Professor Xugang Guo from the Department of Materials Science and Engineering (MSE) at the Southern University of Science and Technology (SUSTech) has led his research team to publish several important papers in high-profile journals such as Journal of the American Chemical Society (JACS), Advanced Materials, Energy & Environmental Science, Angewandte Chemie, and Accounts of Chemical Research.

The first paper, entitled “Cyano-Functionalized Bithiophene Imide-Based n‑Type Polymer Semiconductors: Synthesis, Structure−Property Correlations, and Thermoelectric Performance,” was published in JACS.
Bithiophene imide (BTI)-based semiconductors suffer from high-lying frontier molecular orbital (FMO) energy levels due to their electron-rich bithiophene framework and monoimide group, which is detrimental to n-type performance. To lower the Lowest Unoccupied Molecular Orbital (LUMO) level of BTI, the strong electron-withdrawing cyano groups are attached to the β-positions of the thiophenes in BTI to yield a novel building block CNI.
Considering the potentially increased steric hindrance between the cyano and carbonyl functionalities, two additional thiophenes are fused to BTI to afford CNDTI. Due to the reduced solubility after ring fusion, an unsymmetrical building block CNTI with a small p-conjugated framework is also devised for better solubility.
Based on these novel building blocks, acceptor−acceptor (A-A) type homopolymers and copolymers were successfully synthesized. Their feature significantly suppressed the LUMO energy level (−3.64 to −4.11 eV) versus (−3.48 eV) that of the control polymer PBTI. Their deep-positioned LUMOs resulted in improved stability in organic thin-film transistors (OTFTs) and much more efficient n-doping in organic thermoelectrics (OTEs) for the corresponding polymers with a highest electrical conductivity of 23.3 S cm−1 and a power factor of ∼10 μW m−1 K−2 achieved. The conductivity and power factor are among the highest values reported for solution-processed molecularly n-doped polymers to date.
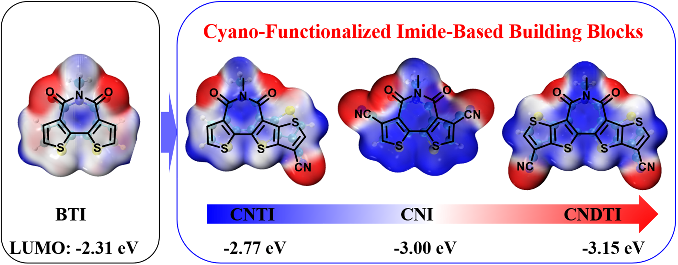
Figure 1. The chemical structure of bithiophene imide and cyano-functionalized bithiophene imide derivatives, and the calculation results of LUMO energy levels.
The second paper, entitled “Regioregular Narrow-Bandgap n-Type Polymers with High Electron Mobility Enabling Highly Efficient All-Polymer Solar Cells,” was published in Advanced Materials.
All-polymer solar cells (all-PSCs) based on polymers as both the electron donor materials and acceptor materials show unique merits versus other types of organic solar cells (OSCs), including superior stability and mechanical robustness. However, it is still important and challenging to develop polymer acceptors with high electron mobilities.
Prof. Guo’s group designed and synthesized two regioregular narrow bandgap polymer acceptors L15 and MBTI (Figure 2), with two electron-deficient segments by copolymerizing two dibrominated fused-ring electron acceptors (FREAs) with distannylated bithiophene imide, respectively (Figure 2). Both polymer acceptors exhibit narrow bandgap and high electron mobility. Benefitting from the more extended absorption, better backbone ordering, and higher electron mobility than its regiorandom analog, L15-based all-PSCs yield a remarkable power conversion efficiency (PCE) of 15.2%.
More importantly, MBTI incorporating a benzothiophene-core FREA segment shows relatively higher frontier molecular orbital levels than L15, forming a cascade-like energy level alignment with L15 and PM6. Based on this, the team designed all-PSCs where MBTI is introduced as a guest into the PM6:L15 host system. Thanks to further optimal blend morphology and more balanced charge transport, the PCE is improved up to 16.2%, which is among the record PCE values for all-PSCs.
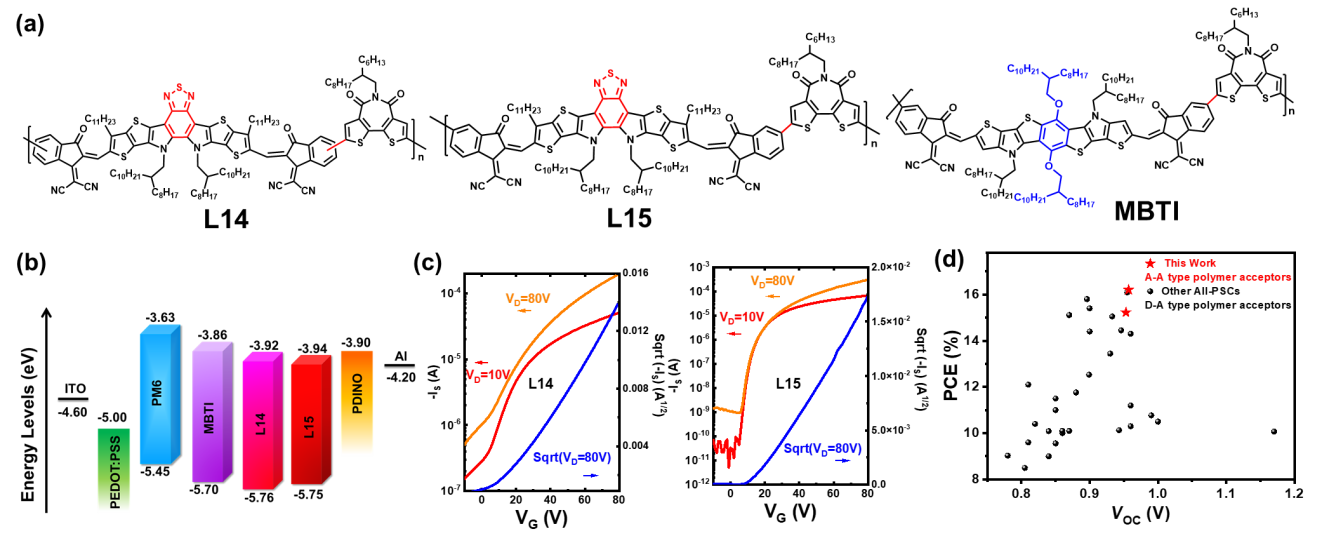
Figure 2. (a) Molecular structures of regioregular A-A type polymer acceptors L15 and MBTI with random analog L14 as the control. (b) Energy level diagram of the materials used in all-PSCs. (c) TG/BC OTFT transfer characteristics of L14 and L15. (d) The plots of PCE against Voc for all-PSCs reported previously with PCEs over 8% from D-A type polymer acceptors and this work from regioregular A-A type polymer acceptors.
The third paper, entitled “Achieving highly efficient all-polymer solar cells by green-solvent-processing under ambient atmosphere,” was published in Energy and Environmental. Prof. Guo’s group has made a breakthrough in the research of green solvent processing all-polymer solar cells and the relevant result in Science.
They used BTI-based narrow bandgap n-type acceptor L14 blended with the common polymer donor PM6 as the active layer (Figure 3). The halogen-free processing solvents are utilized for fabricating eco-friendly and highly-efficient all-PSCs. In particular, o-xylene solvent-processed all-PSCs achieve a high PCE of 15.6%, which is the highest value among the green-solvent-processed all-PSCs to date.
Detailed investigations reveal that such enhancement is mainly attributed to the optimal blend morphology and polymer crystalline structure resulting from aggregated structures of polymers in fast-evaporation of o-xylene, which controls the kinetics and thermodynamics of all-polymer blend film formation. Importantly, the o-xylene-processed-all-PSCs can be fabricated in ambient conditions due to the use of green-solvent, affording a high PCE approaching 15.0 %.
This work highlights the importance of green solvent strategy in optimizing the polymer aggregated structures and blend morphology of all-PSCs, paving the way towards high-performance and eco-friendly all-PSCs for practical applications.
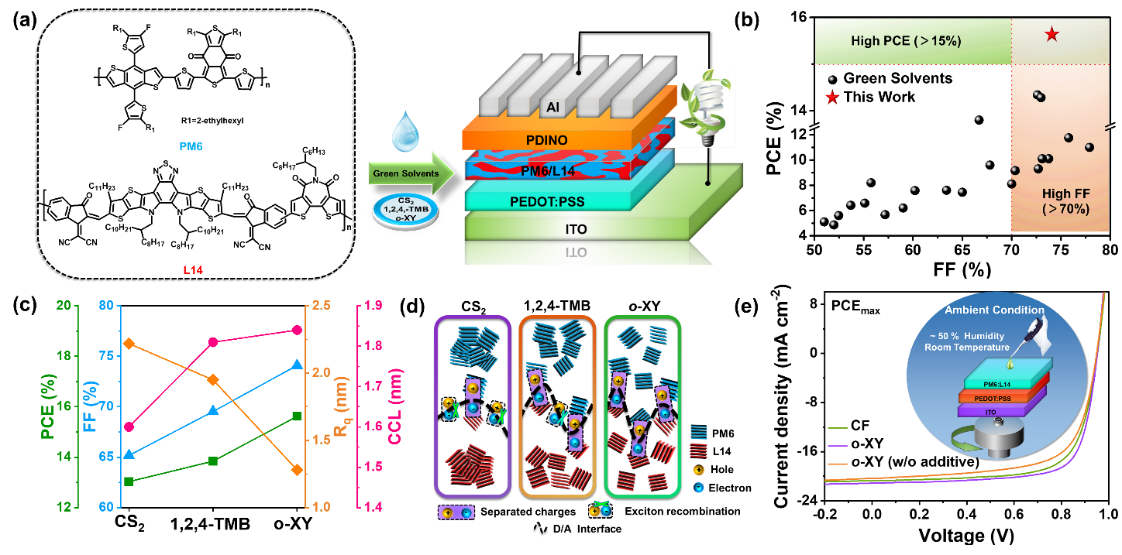
Figure 3. (a) Chemical structures of PM6 and L14 and the corresponding device structure used in this work. (b) Plots of the PCE versus FF for green-solvent-processed all-PSCs with PCEs of over 4 % reported in the literature. (d) Device parameters, RMS roughness, and Lc values of the OOP (010) peaks versus processing green solvents. (e) The D/A interfaces schematic diagram of PM6: L14 active layer processed by various green solvents.
The fourth paper, entitled “Fused Bithiophene Imide Dimer-Based n-Type Polymers for High-Performance Organic Electrochemical Transistors,” was published in Angewandte Chemie.
The development of n-type (electron-transporting) polymers in OECTs lags considerably behind with the product (µC*) of charge carrier mobility (µ) and volumetric capacitance (C*) values typically in the range of only 0.1–1 F cm–1 V–1 s–1. The mismatch between n-type and p-type performance restricts the development of low-power OECT-based complementary circuits for in situ amplification in bioelectronic applications.
The team developed two D-A type polymers, f-BTI2TEG-T and f-BTI2TEG-FT, comprising fused bithiophene imide dimer (f-BTI2) bearing oligo(ethylene glycol) side chain as the acceptor unit and thiophene/difluorothiophene as the donor co-unit. The fluorinated f-BTI2TEG-FT shows a low-lying LUMO level and substantial electrochemical doping efficiency in an aqueous environment. When applied in OECTs, the f-BTI2TEG-FT-based devices achieved an unprecedented geometry-normalized transconductance (gm,norm.) of 4.60 S cm–1 and µC* of 15.2 F cm–1 V–1 s–1, which are the highest values reported to date for n-type OECTs.
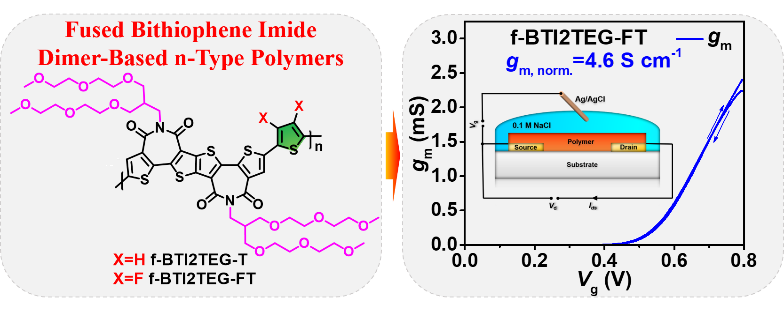
Figure 4. The chemical structure of n-type polymer f-BTI2TEG-T and f-BTI2TEG-FT, and the transfer characteristic of f-BTI2TEG-T-based OECTs.
The pioneering and systematic works of Prof. Xugang Guo on n-type organic and polymeric semiconductors have been well recognized by his peers worldwide. It includes all this work from the first naphthalene diimide (NDI)-based polymer to the recently developed bithiophene imide-based organic and polymer semiconductors. He and his research team were invited to write a review about bithiophene imide derivatives.
The fifth paper, entitled “Organic and Polymeric Semiconductors Based on Bithiophene Imide Derivatives,” was published in the high-impact academic journal Accounts of Chemical Research from the American Chemical Society. It summarizes the significant material progress, device applications, and challenges of bithiophene imide derivative-based n-type semiconductor materials in recent years.
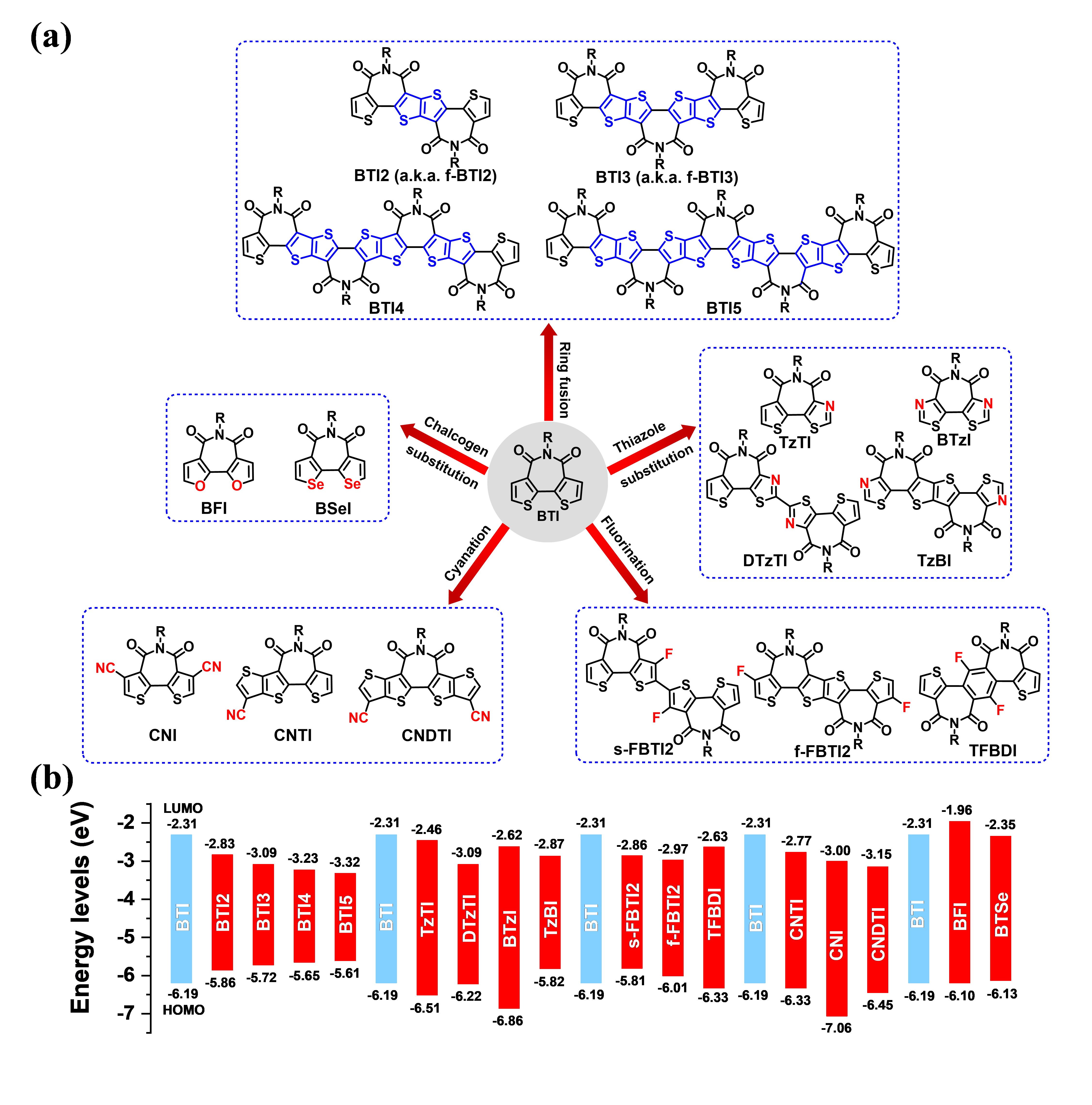
Figure 5. (a) Chemical structures of electron-deficient building blocks derived from bithiophene imide (BTI) via various strategies. (b) Calculated FMO levels of BTI and its derivatives.
The research works above were in collaboration with Professor Han Young Woo’s team of Korea University, Professor Li Niu’s team of Guangzhou University, Professor Qingdong Zheng’s team of Fujian Institute of Research on the Structure of Matter of Chinese Academy of Sciences (FJIRSM, CAS), Professor Bumjoon J. Kim’s team of Korea Advanced Institute of Science and Technology (KAIST), Professor Hong Meng’s team of Peking University Shenzhen Graduate School (PKU Shenzhen), and Professor Simone Fabiano’s team of Linköping University (LiU), Sweden. The researchers would also like to acknowledge the SUSTech Core Research Facilities for its assistance with the material and device characterizations.
Paper links (In order of appearance above):
JACS: https://dx.doi.org/10.1021/jacs.0c11608
Advanced Materials: https://doi.org/10.1002/adma.202102635
Energy & Environmental Science: https://doi.org/10.1039/D1EE01310F
Angewandte Chemie: https://doi.org/10.1002/anie.202109281
Accounts of Chemical Research: https://doi.org/10.1021/acs.accounts.1c00381
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo By