Axon guidance is a subfield of neural development that examines the process that neurons send out nerve cells to their intended targets. Axons often follow exceptionally precise paths within the nervous system, and these paths have been the inspiration of substantial research. The improved understanding of these pathways could improve our understanding of how the human brain works.
Associate Professor Ji Shengjian from the Department of Biology at Southern University of Science and Technology (SUSTech) led his research group to publish a paper in renowned journal Nucleic Acids Research. The paper was titled as “The m6A reader YTHDF1 regulates axon guidance through translational control of Robo3.1 expression.” The paper examined the role of m6A modification in regulating axon guidance.
m6A, also known as N6-Methyladenosine, is the most widely distributed internal modification in mRNA. m6A modification of mRNA is a dynamic and reversible process.
Robo3 is thought to be required for commissural axons to cross the floor plate, a structure that is integral to the development of the nervous system of vertebrate organisms. Whether the neuron crosses the midline of a vertebrate or not is determined by the floor plate. When the neuron crosses the midline and guides to the relevant target, the embryo will be able to develop successful coordination of left and right body halves.
Their study showed that ablation of Ythdf1 in spinal commissural neurons results in pre-crossing axon guidance defects. It also identified a mechanism for YTHDF1-mediated translation of m6A-modified Robo3.1 mRNA that would control pre-crossing axon guidance in the spinal cord.
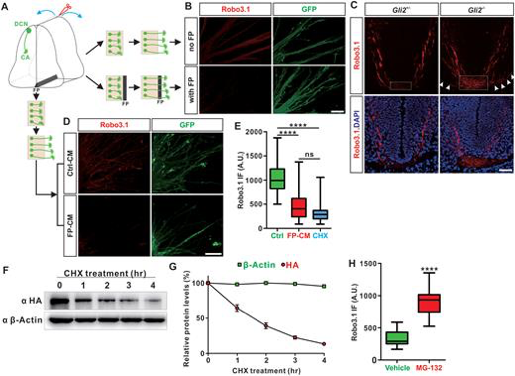
The research team found that the Robo3.1 protein has a short half-life and maintenance of its protein levels requires continuous translation of Robo3.1 mRNA. The floor plate controls the elimination of Robo3.1 protein.
The research team acknowledged existing studies that show the role m6A modification plays in neuron development and regeneration. However, how m6A modification works and what are the neuronal target mRNAs for m6A readers remain to be further investigated. The team recommends future research into the identification of m6A-modified neural mRNAs targeted by YTHDF1 (and other m6A readers as well) at the transcriptomic level. They plan to study the characterization of these mRNAs in order to better understand the functions and mechanisms of m6A modification in the nervous system.
SUSTech was the first affiliation of all authors, with the research completed by Ji Shengjian’s research group. Zhuang Mengru, a SUSTech-HKUST Joint PhD program candidate, is the first author, and SUSTech undergraduate Li Xinbei is the second author. Associate Professor Ji Shengjian is the corresponding author.

The research group received funding from the Natural Science Fund of Guangdong Province, Basic Research Grant and Technology Innovation Grant of Peacock Plan from the Shenzhen Municipal Science and Technology Innovation Commission. The running cost of Associate Professor Ji Shengjian’s laboratory was covered by startup funds from SUSTech and Peacock Plan of Shenzhen Municipal Government.
Proofread By
Photo ByDepartment of Biology