Recently, Yongye Liang’s research group in SUSTech developed a new molecular fluorophore for bioimaging in the second near infrared (NIR-II) window. In collaboration with Prof. Hongjie Dai’s group in Stanford University and researchers from School of Medicine, they successfully applied this fluorophore for noninvasive NIR-II imaging of the mouse’s brain to investigate cerebrovascular injury in a mouse model of traumatic brain injury (TBI). This work has been published in the journal “Advanced Materials” entitled “Traumatic Brain Injury Imaging in the Second Near-Infrared Window with a Molecular Fluorophore”.
Fluorescence imaging in NIR-II window (fluorescent emission in the 1,000-1,700 nm range) is useful for non-invasive in vivo imaging at sub-centimeter tissue depth with sub-10 micron spatial resolution. Because of the reduced photon scattering and auto-fluorescence by biological tissues at longer wavelengths, NIR-II can achieve deeper and better imaging than conventional NIR-I window (750-900 nm). A bottleneck for the biomedical imaging in NIR-II window is the lack of good fluorophores. Inorganic nanomaterials, such as carbon nanotubes, Ag2S quantum dots, and rare earth nanoparticles have been employed as NIR-II fluorophores, but these nanomaterial fluorophores are generally very difficult to be cleared out from the body due to their large size and have toxicity concerns. Recently, a molecular dye has been reported with NIR-II emission and demonstrated renal excretion. However, the emission quantum yield of such dye is too low (less than 0.2%) for real-time imaging, and the fluorescence is limited below ~1200 nm.
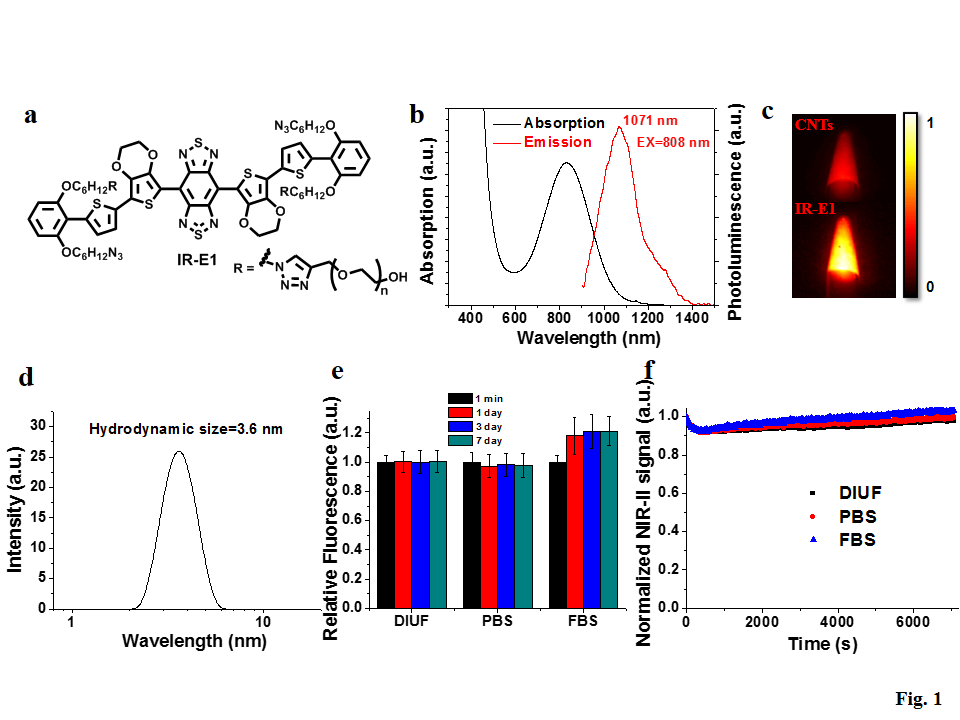
Liang’s group proposed a new molecular fluorophore design with the introduction of enveloping group and the use of 3,4-ethoxylene dioxythiophene (EDOT) as donor, which could reduce intermolecular and intramolecular interactions and improve the fluorophore quantum yield in aqueous solution. Click reaction was employed to introduce PEG chain for high aqueous solubility. The synthesized fluorophore, IR-E1, exhibited emission from 900 nm to 1400 nm with quantum yield of 0.7 % (2X of carbon nanotube) in water and excellent aqueous and photo-stability (Figure 1). IR-E1 also demonstrated good biocompatibility and fast renal excretion of > 80% within 24 hours post injection.
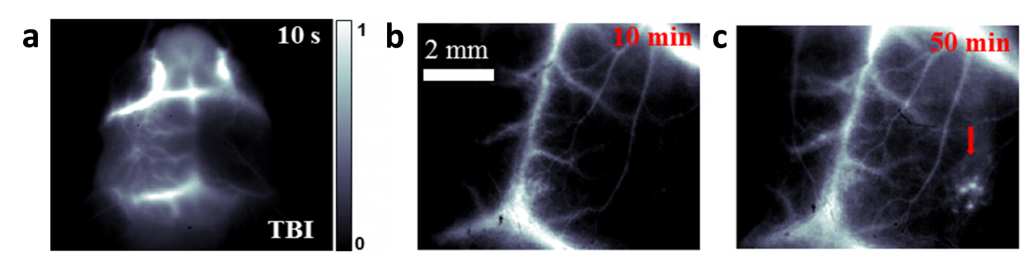
The high performance of IR-E1 allowed noninvasive in vivo imaging of cerebrovascular injury in a mouse model of TBI through tail vein intravenous injection of the NIR-II fluorophore into mouse (Figure 2). The dynamic neurovascular changes were characterized with high-spatial and temporal resolution, and cerebral hypoperfusion, fluorophore leakage and trapping in the injured brain tissue were directly visualized. This study could afford new opportunities for probing TBI pathogenesis and devising therapeutic approaches.
Huasen Wang from Liang’s group and Xiaodong Zhang from Dai’s group are the first authors with equal contributions. A senior undergraduate in SUSTech, Rui Ma, also participated in this work. Other coauthors include X. D. Zhang, A. L. Antaris, S. Diao, Dr. G. Hong, Z. Ma, J. Wang, Dr. S. Zhu, Prof. H. Dai from Department of Chemistry, Stanford University, and Dr. L. Li, A. Nguyen, J. M. Castellano, T. Wyss-Coray, J. Luo from School of Medicine, Stanford University and VA Palo Alto Health Care System. The work was supported by “Recruitment Program of Global Experts”, basic research foundation, key laboratory program and Peacock program of Shenzhen, and startup support (FRG-SUSTC1501A-62) from SUSTech).
Proofread By
Photo By