There is significant research into new materials for fuel cells that directly electro-oxidize formic acid into carbon dioxide, but the development of high noble metal mass‐normalized activity and long‐term stability catalysts remain a significant challenge for the commercialization of direct liquid fuel cells.
The efforts of Professor Quan Zewei and his research group from the Department of Chemistry at Southern University of Science and Technology has seen their research into unique intermetallic compounds published in Advanced Materials, in a paper titled “Trimetallic Synergy in Intermetallic PtSnBi Nanoplates Boosts Formic Acid Oxidation.”
The traditional formic acid catalyst of platinum (Pt) has poor catalytic properties due to the electrochemical reaction generating carbon monoxide (CO) caused by the indirect oxidation path of formic acid, on the direct way to forming carbon dioxide (CO2). There have been advances in this field of chemistry, but they lack the commercial qualities for the further development of formic acid fuel cells.
It is well known that the formation of CO intermediate from the oxidation comes primarily from three or more contiguous Pt atomic sites. On the other hand, creating isolated Pt sites could suppress the formation of CO intermediate, thereby dramatically improving the direct oxidation performance of formic acid.
With that in mind, the development of Pt-based intermetallic compounds (alloys with well‐defined composition and long‐range atomic ordering) has been the source of considerable research due to their properties that support superior catalytic performance. There are further opportunities to optimize the catalytic performance of Pt-based intermetallic compounds by using different multi-metallic, but these are very challenging to form and research into their reactions with formic acid are highly limited.
The research group used a simple method to prepare a new class of ternary intermetallic nanoplates, made up of platinum, tin and bismuth (PtSnBi). This particular formation was showed to have a uniform and orderly distribution with a significantly higher catalytic performance than binary PtBi or PtSn intermetallic compounds. Specially, the atomically ordered Pt45Sn25Bi30 nanoplates, exhibits an ultrahigh mass activity of 4394 mA mg−1 Pt and preserves 78% of the initial activity after 4000 potential cycles, which make it a state‐of‐the‐art catalyst toward formic acid oxidation. They also found that the electronic and structural properties of PtSnBi nanoplates inhibit the intermediate development of CO and optimize the creation of CO2, thereby significantly improving the direct oxidation performance of formic acid. As a result, there is a new path for the development of multi-metal intermetallic compounds for highly efficient catalysis.
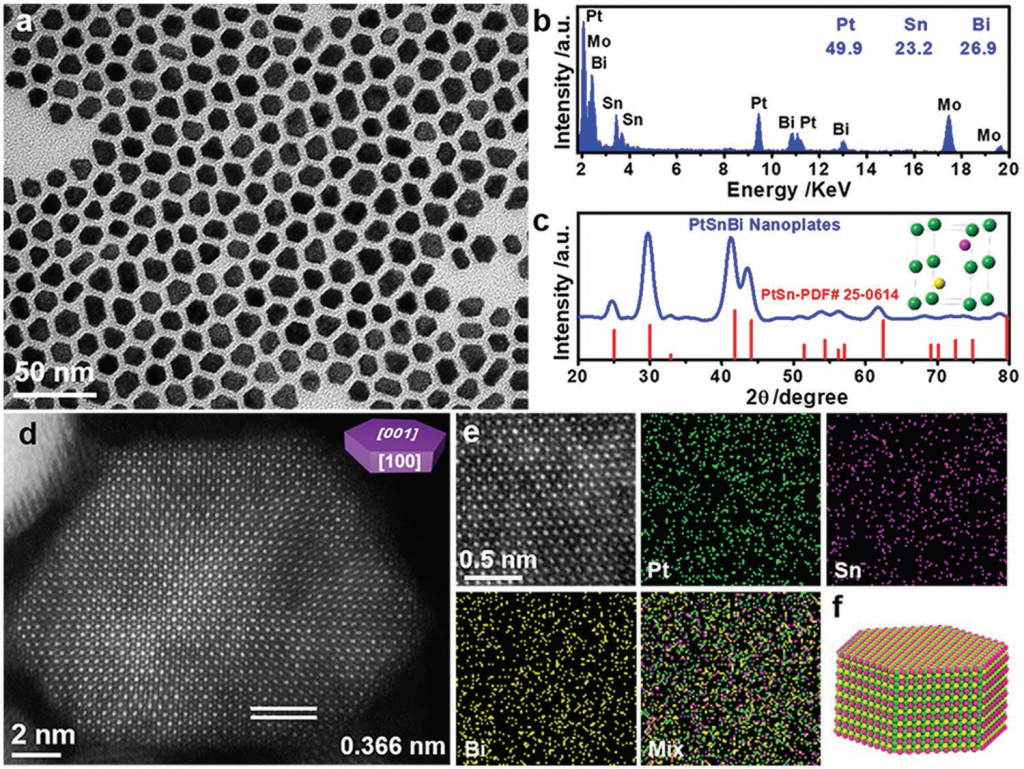
Figure 1. Structural characterization of Pt45Sn25Bi30 ternary intermetallic compound nanoplates
In the research work, SUSTech is the only unit, Professor Quan Zewei is the correspondent author, and post-doctoral researcher Luo Shuiping is the first author of the paper. Doctoral student Chen Wen and Class of 2020 student Cheng Yu are significant contributing authors to the paper.
The work was supported by the National Natural Science Foundation of China, the Shenzhen Science and Technology Innovation Commission, the Development and Reform Commission of Shenzhen Municipality, Novel Nanomaterial Discipline Construction Plan, the SUSTech Presidential Fund, and the China Postdoctoral Science Foundation.
Proofread ByXia Yingying
Photo ByDepartment of Chemistry