Research at Southern University of Science and Technology has found a new target for cancer treatment with a unique approach to deal with the degradation of protein within cells that cause cancer.
Earlier this month, Associate Professor Rao Feng from the Department of Biology at Southern University of Science and Technology published a paper in conjunction with his former colleague Wang Tao, who is now at the Shenzhen Bay Laboratory. Their paper was published in the Proceedings of the National Academy of Sciences of the United States of America (PNAS) under the title of “Basis for metabolite-dependent Cullin-RING ligase deneddylation by the COP9 signalosome.” On publication, it was recommended by F1000 as a paper of special significance. Subsequent to its publication, PNAS published a Commentary on the paper, discussing its significance.
Cancer cells are formed when the actions of ubiquitylation enzymes erroneously degrade proteins executing cell death or senescence. The biggest family of those enzymes is known as Cullin RING E3 Ligases (CRL). Pevonedistat, a CRL inhibitor, is undergoing a phase III anti-cancer clinical trial.
Current research has also found that the enzyme that specifically inactivates CRL is called the COP9 signalosome (CSN). However, there is a lack of understanding of how CSN recognizes CRL and how it could be used in different ways to inhibit CRL.
The paper published by Associate Professor Rao Feng found that a metabolite known as inositol hexakisphosphate (IP6) is essential in binding CRL and CSN together, which ensures that CRL is not activated. Using a combination of biochemistry, structural biology (x-ray crystallography and Cryo-EM map fitting), chemical biology, and genetics approaches, the authors found that this abundant intracellular metabolite directly interacts with CRL and pulls it into proximity with CSN.
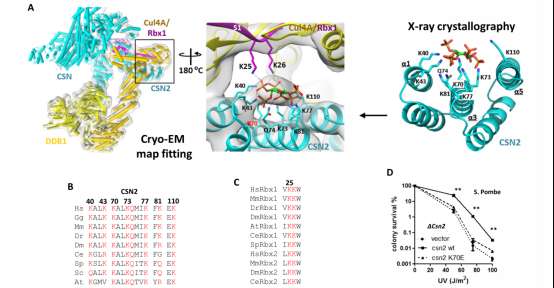
Figure 1. The structure of the IP6-CSN-CRL ternary complex and its evolutionary conservation from yeast to human.
IP6 has been found in fundamentally the same evolutionary structure from yeast to plants to humans. It indicates that it is essential for all forms of life, including for CRL-CSN regulation.
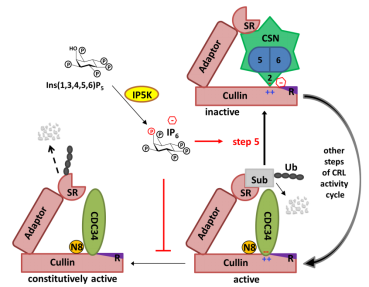
Figure 2. Schematic diagram of the dynamic regulation of CRL ubiquitin ligase depigmentation by metabolic small molecule IP6.
The research explains the formation of the IP6-CSN-CRL complex at the molecular level, and further suggest that targeting IP6 biosynthesis or the IP6-binding pocket at CRL-CSN interface could be used to treat cancer in the future through inactivating CRL.

The work was completed in the laboratory of Associate Professors Rao Feng and Wang Tao at SUSTech, who are also the corresponding authors. The three co-first authors were research scholar Lin Hong and graduate student Zhang Xiaozhe from the Rao lab, and postdoctoral researcher Liu Li from the Wang lab, all in the Department of Biology. Additional support came from investigator Dr. Zheng Ning from the Howards Hughes Medical Institute at the University of Washington, Drs. Huang Niu & Du Li-Lin from the National Institute of Biological Sciences in Beijing, and Professor Zhou Chuanzheng at the State Key Laboratory of Elemento-Organic Chemistry at Nankai University.
This research was supported by the National Science Foundation of China (NSFC), the Shenzhen Municipal Science and Technology Innovation Commission, the Guangdong Provincial Department of Science & Technology, the Ministry of Science and Technology, and the NSFC-Guangdong Joint Fund.
Proofread ByRao Feng
Photo ByDepartment of Biology