On January 25, 2021, the research team of Xumu Zhang, Vice Dean of the College of Science and Professor of the Department of Chemistry at the Southern University of Science and Technology (SUSTech), and Professor Deyin GUO, Dean of the School of Medicine at Sun Yat-Sen University, as well as Professor Yu Zhang, Deputy Director of Guangdong Laboratory Animal Monitoring Institute, published an original research paper titled “Remdesivir Metabolite GS-441524 Effectively Inhibits SARS-CoV-2 Infection in Mouse Models” in the Journal of Medicinal Chemistry.
Figure 1.
The global pandemic caused by the SARS-CoV-2 has brought a significant impact on various fields around the world. According to the International Monetary Fund (IMF), the global economy will shrink by 4.4% in 2020 (a loss of $11 trillion), which is the worst recession since World War II. The loss might be up to $28 trillion between 2020 and 2025. Without a new drug or vaccine that would prevent the spread of SARS-CoV-2, the threat of the virus will remain, and the global economic recovery will still be unsteady. Before publication, Remdesivir, originally developed by Gilead Sciences Inc. (GILD) for treating the Ebola virus, was the only FDA-approved drug for SARS-CoV-2 infections. However, the clinical results of the WHO solidarity trial of Remdesivir did not show a clear difference in mortality, which raised many questions about its effectiveness. In addition, the widespread use of the drug was affected by its form of administration with only intravenous (IV) injection rather than oral medication. Therefore, the rapid development of an orally available drug with improved efficiency will be an essential and urgent task. Therefore, Professor Xumu Zhang’s team carried out extensive research on anti-SARS-CoV-2 drug development.
First of all, the team compared the anti-SARS-CoV-2 activity of the metabolite of remdesivir, GS-441524 (parent nucleoside), and remdesivir in Vero E6 (African green monkey kidney), Calu-3 (human lung adenocarcinoma), and Caco-2 (colorectal adenocarcinoma) cell lines. The in vitro studies showed that GS-441524 and remdesivir effectively inhibited SARS-CoV-2 replication in a dose-dependent manner without affecting cell proliferation. The cytotoxicity test showed that the GS-441524 treatment did not decrease the cell viability at 50 μM, indicating that GS-441524 treatment was safe at this concentration.
After the pharmacokinetic study of GS-441524 in rats, the therapeutic effect of GS-441524 on SARS-CoV-2 infection was evaluated in the AAV-HACE2 mouse model. Mice treated with GS-441524 demonstrated significant virus clearance in the lungs at 2 days post-inoculation (dpi) and did not experience weight loss. Lung tissue was taken 10 days after infection, and pathological sections showed a reduction in inflammatory cell infiltration from the trachea and alveoli to the interstitium. Together, this data (Fig. 2) provides the first solid demonstration of the efficacy of GS-441524 against SARS-CoV-2 in vivo.
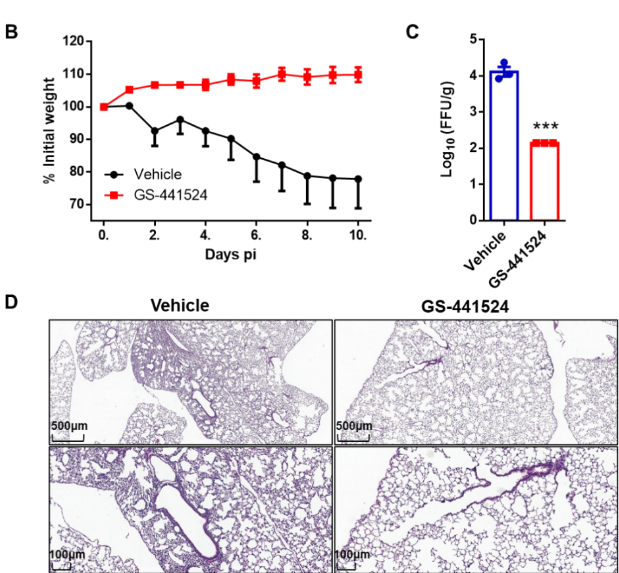
Figure 2. Anti-SARS-CoV-2 efficacy of GS-441524 in an AAV-hACE2 mouse model. (B) Changes in body weight for either vehicle (black) or GS-441524-treated (red) mice. (C) Viral titers from lung tissue of 3 mice per group were harvested at 2 dpi and analyzed by focus forming assay (FFA). ***p-value ≤ 0.0005. (D) Representative hematoxylin-eosin (H&E) staining of lungs from hACE2 transduced mice. Scale bars, 500 μM (top) and 100 μM.
Lastly, the team tested the efficacy of GS-441524 on mice infected with MHV (murine hepatitis virus). The study showed that GS-441524 could effectively reduce the viral load in the liver. The survival rate was 100% when the drug was given orally or through IV. Compared with the 12.5% survival rate in the control group, the mortality rate of mice was significantly reduced, and the drug did not cause significant weight loss. The results indicated that GS-441524 has a remarkable anti-MHV activity and a safety profile (Fig. 3).
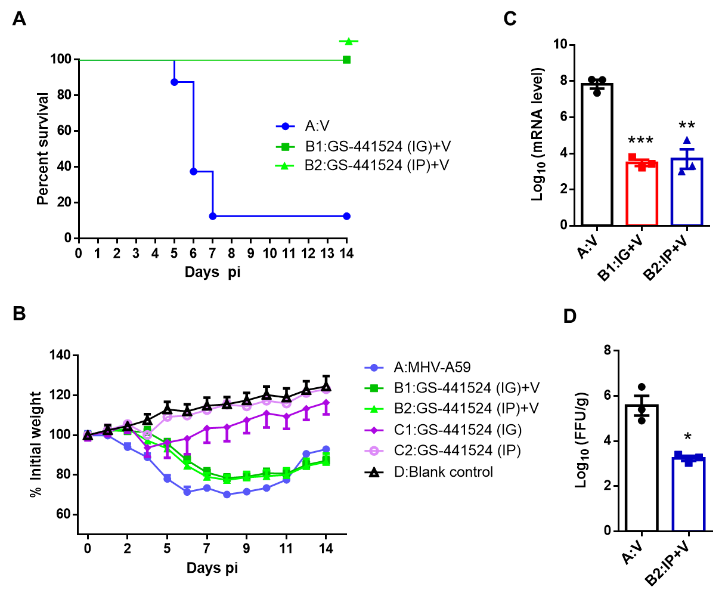
Figure 3. Antiviral efficacy of GS-441524 in mice with MHV-A59. (A) Survival curves of mice in Groups A (blue) B1 (dark green) and B2 (light green). (B) Body weights of animals in the 6 groups. (C) Viral titers in the liver of mice from Group A (black), Group B1 (red), and Group B2 (blue) quantified by qRT-PCR at 3 dpi. (D) Viral titers in the liver of mice from Group A (black) and Group B2 (blue) were quantified by focus forming assay at 3 dpi.
Although remdesivir has been approved by the FDA to treat COVID-19, questions remain about its efficacy. Due to its long synthetic route, the high price, and the IV administration, remdesivir’s use and accessibility will be limited. Structurally, remdesivir, as a prodrug of McGuigan nucleoside, hydrolyzed in vivo to the monophosphate and then phosphorylated in two steps to obtain the active triphosphate. The initial conversion of nucleosides, such as GS-441524, to a monophosphate, is considered as a rate-limiting step. However, GS-441524 can be effectively converted into the active phosphorylated form in cells, according to the similar antiviral activities of these two compounds. In conclusion, GS-441524 is superior to remdesivir in the treatment of COVID-19 in the following two aspects:
1. As compared to remdesivir, GS-441524 has the advantages of simple structure, short synthesis steps, and low cost, making it suitable for rapid mass production as an emergency drug.
2. The clinical pharmacokinetic study of remdesivir showed that the metabolite GS-441524 had a long half-life of about 27 hours in the human body. The results from this study support evidence of the early safety and efficacy of GS-441524 against COVID-19 and other coronavirus infections and highlights the need to conduct clinical trials for the treatment of COVID-19.
Professor Xumu Zhang said that based on the above research results and the efficient SUSTech platform for small molecule synthesis, the team has explored the development of new orally effective RdRp inhibitor drugs. A series of nucleoside analogs have been designed and synthesized. The preclinical study of one of the active compounds, named “SHEN26”, is ongoing as a candidate for the treatment of COVID-19.
Yingjun Li, an associate research fellow of SUSTech, and Liu Cao, a postdoctoral fellow of the School of Medicine at Sun Yat-Sen University, are co-first authors of this paper. Professor Xumu Zhang, Vice Dean of the College of Science at SUSTech, Professor Deyin Guo, Dean of the School of Medicine at Sun Yat-Sen University, and Professor Yu Zhang, Deputy Director of Guangdong Laboratory Animals Monitoring Institute are co-corresponding authors of this paper. SUSTech was the first affiliation of the article.
This project is financially supported by the Shenzhen Science and Technology Innovation Committee, Shenzhen Bay Laboratory, the National Natural Science Foundation of China, as well as support from the Science and Technology Department of Guangdong Province and Guangdong Laboratory Animals Monitoring Institute. The project is now an important research achievement in the field of antiviral drug development for COVID-19. It provided a significant amount of scientific foundation and technical support for the team to conduct more precise and comprehensive drug research in the future at SUSTech.
Paper link:https://pubs.acs.org/doi/10.1021/acs.jmedchem.0c01929
Proofread ByAdrian Cremin, Yingying XIA
Photo By