On February 10th, the research teams of Assistant Professor Tiezheng Jia from the Department of Chemistry at the Southern University of Science and Technology (SUSTech) and Professor Marisa Kozlowski from the Department of Chemistry at the University of Pennsylvania (UPenn) collaborated to publish a paper in the high-impact academic journal, Nature Communications. Their paper was entitled “Autocatalytic photoredox Chan-Lam coupling of free diaryl sulfoximines with arylboronic acids.”
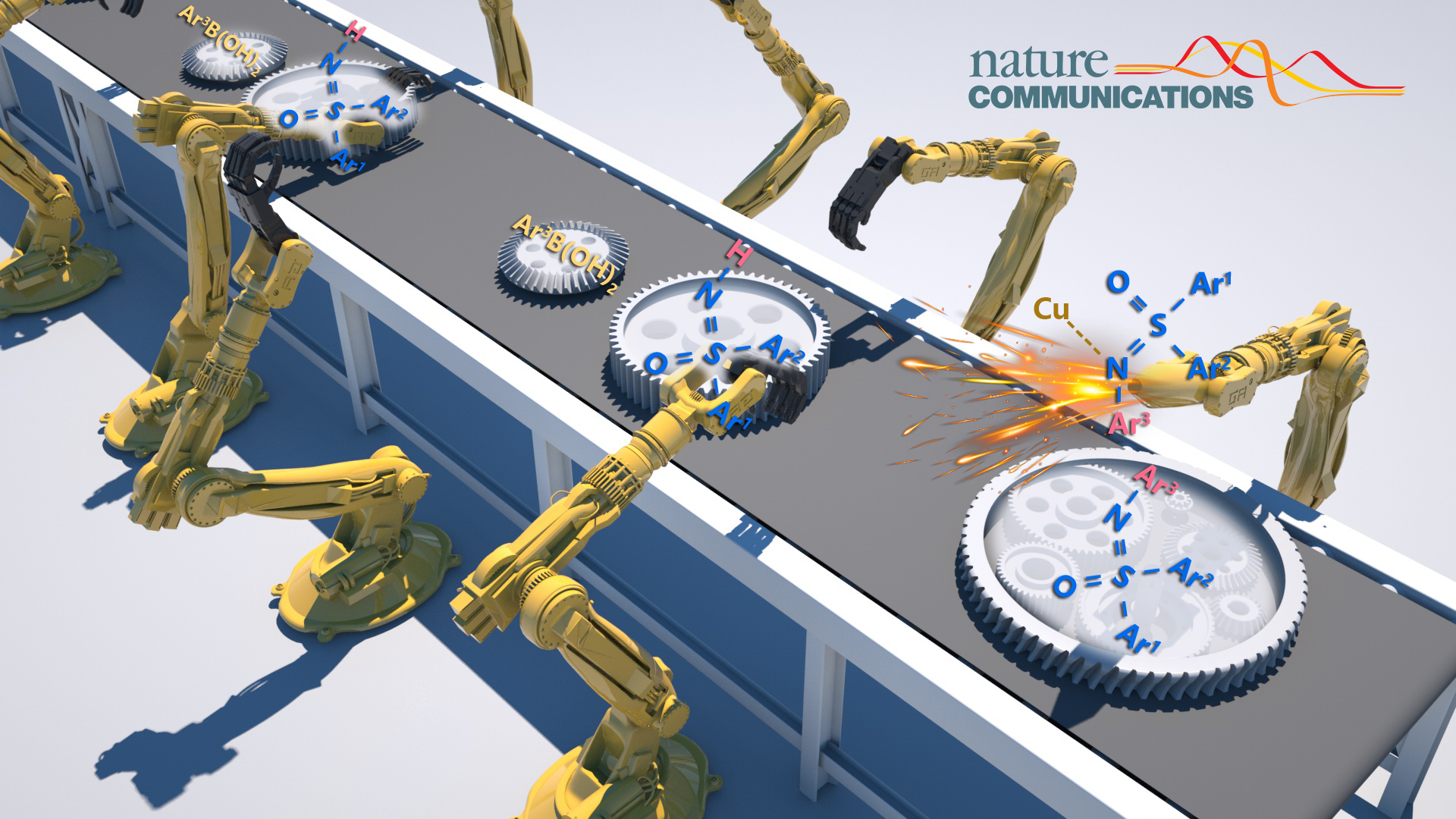
This work used copper(II) trifluoroacetate hydrate as the copper source, ethanol as solvent, and introduced a photoredox copper-catalyzed dehydrogenative Chan-Lam coupling of free diaryl sulfoximines and arylboronic under ambient light. A series of N-aryl sulfoximines were constructed with high yield and good substrate compatibility.
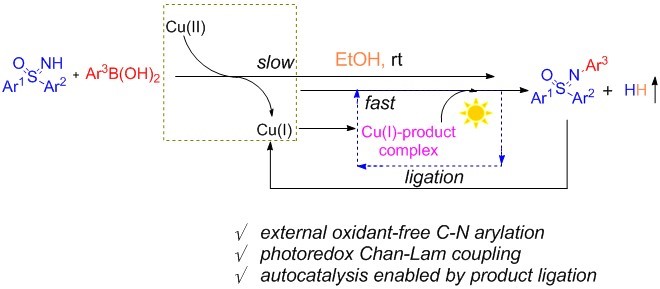
Figure 1. Autocatalytic photoredox Chan-Lam coupling
The mechanism was studied by control experiments and DFT calculations. Mechanistic investigations reveal a unique autocatalysis process (Figure 2). Initially, a copper(II)-mediated C–N coupling occurs between free sulfoximine and arylboronic acid to give a small amount of N-aryl sulfoximines. The ligation of N-arylated sulfoximines to copper species enables its capability as a photocatalyst, which efficiently facilitates the C–N coupling process in the absence of external oxidant.
During this catalytic process, there is a key mixed species (E’), which is believed to facilitate the photocatalytic process. The calculation shows that the intermediate (E’) possesses lower energy than D’, which can be generated from C’ or D’ via a ligand exchange process at room temperature (Figure 3) that further supports the hypothesis.

Figure 2. Proposed mechanism

Figure 3. Thermodynamic cycles of substrate/product ligand exchange for Cu(I) and Cu(II)
This study helps to understand the autocatalytic properties and the unique ligand-promoted photoexcitation of copper species. The protocol described herein represents an appealing alternative strategy to the classic oxidative C–N coupling process, allowing the expansion of substrate generality as well as the elimination of byproducts from catalyst oxidation. The concept of sacrificial oxidant-free photoredox Chan-Lam coupling provides a basis for the design of systems to allow the formation of C–N bonds in other contexts.
Ph.D. student Cong Wang and Dr. Hui Zhang from SUSTech and Ph.D. student Lucille Wells from UPenn are the co-first authors of this paper. Assistant Professor Tiezheng Jia from SUSTech and Professor Marisa Kozlowski from UPenn are the co-correspondent authors. Additional contributions came from doctoral student Tingting Meng (SUSTech), Professor Qingchao Liu (Northwest University), master student Tian Liu (Northwest University), and Professor Patrick Walsh (UPenn).
The authors received support from the Shenzhen Nobel Prize Scientists Laboratory Project, the Science, Technology and Innovation Commission of Shenzhen Municipality, the Guangdong Provincial Key Laboratory of Catalysis, Open funding Project of the State Key Laboratory of Elemento-Organic Chemistry at Nankai University, and the US National Institutes of Health (NIH).
Paper link:https://www.nature.com/articles/s41467-021-21156-w
Proofread ByAdrian Cremin, Yingying XIA
Photo By