Recently, Dr. Hailiang Hu’s research group from the School of Medicine at the Southern University of Science and Technology (SUSTech) and Dr. Jiaoti Huang’s research group from the Department of Pathology at Duke University, jointly published a research article entitled “A glutaminase isoform switch drives therapeutic resistance and disease progression of prostate cancer” in the top international academic journal PNAS.
The study comprehensively explored the underpinning of the failure of hormonal therapy for prostate cancer and discovered a molecular mechanism by which glutamine metabolism reprogramming can drive the progression of prostate cancer and the development of treatment resistance. These findings provide a novel metabolic target for the diagnosis and treatment of prostate cancer with significant implications in translational medicine research and broad applications for the development of metabolic drugs.

Figure 1. Title page of the PNAS article
Cellular metabolism in cancer is significantly altered to support uncontrolled tumor growth. How metabolic alterations contribute to hormonal therapy resistance and disease progression in prostate cancer remains poorly understood. This study reports a glutaminase isoform switch mechanism that mediates the initial therapeutic effect but the eventual failure of hormonal therapy of prostate cancer.
Androgen deprivation therapy inhibits the expression of kidney-type glutaminase (KGA), a splicing isoform of glutaminase 1 (GLS1) up-regulated by androgen receptor (AR), to achieve the therapeutic effect by suppressing glutaminolysis. Eventually, the tumor cells switch to the expression of glutaminase C (GAC), an androgen-independent GLS1 isoform with more potent enzymatic activity, under the androgen-deprived condition. This switch leads to increased glutamine utilization, hyperproliferation, and aggressive behavior of tumor cells. Pharmacological inhibition or RNA interference of GAC shows better treatment effects for castration-resistant prostate cancer than for hormone-sensitive prostate cancer, suggesting a new avenue for targeting therapy-resistant prostate cancer.
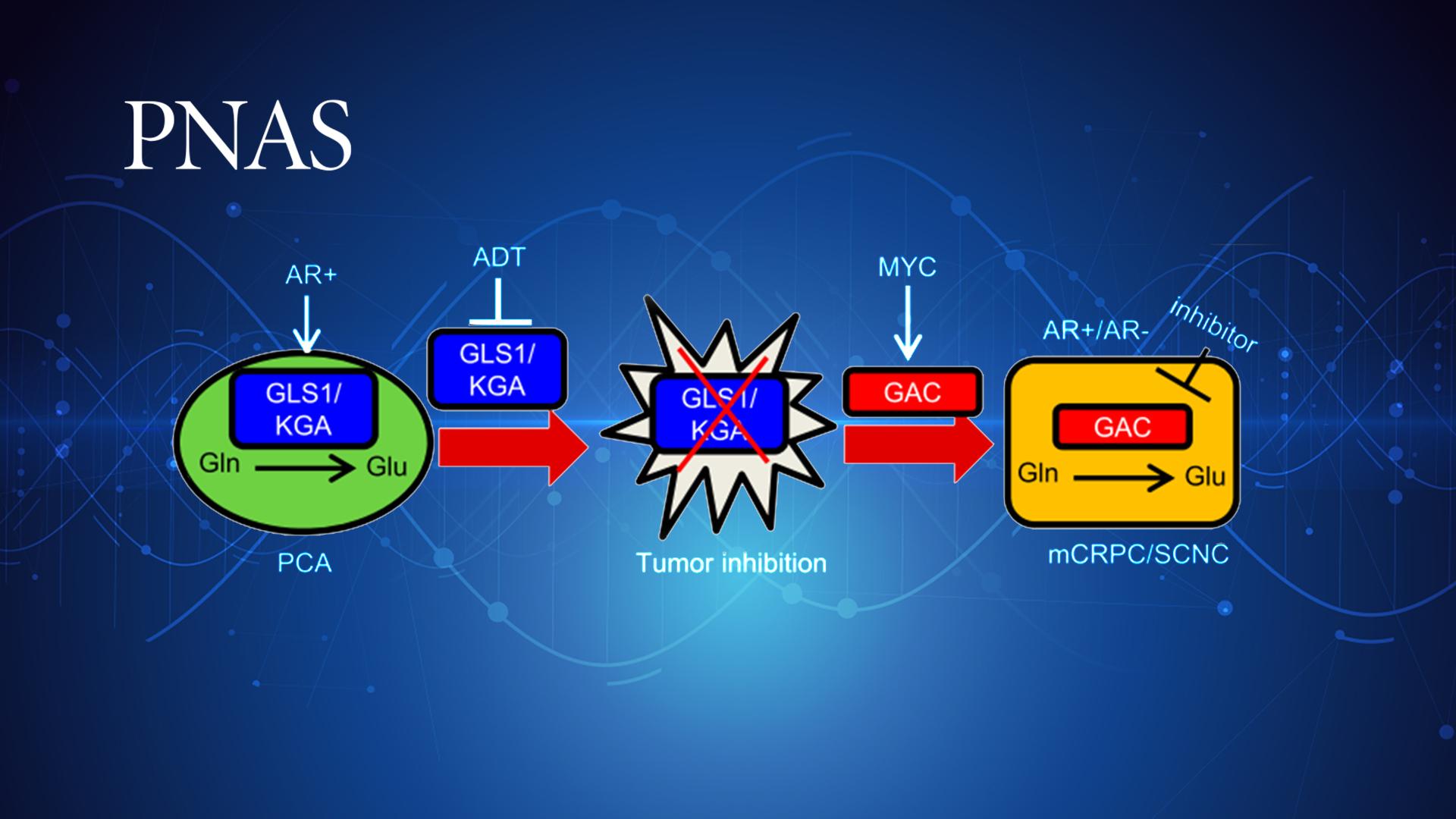
Figure 2. Working model of this research
In summary, this study identified a new metabolic mechanism of the glutaminase isoform switch that drives the therapeutic resistance and disease progression. It has tremendous potential to promote the understanding of the resistant mechanisms and facilitate drug development for the deadly form of prostate cancer.
Associate Professor Hailiang Hu of SUSTech and Professor Jiaoti Huang of Duke University are the co-corresponding authors of this research. Important key collaborators include Drs. Daniel George and Jason Locasale of Duke University, Dr. Xuesheng Dong of the University of British Columbia, and Dr. Dean G. Tang of the Roswell Park Cancer Center.
Dr. Hu’s research group at SUSTech is currently recruiting post-doctoral fellows and graduate students who will work on tumor metabolism, targeted therapy, and DNA damage repair. For further details, please see the link below.
Paper link: https://www.pnas.org/content/118/13/e2012748118
Website link for Dr. Hailiang Hu: https://www.sustech.edu.cn/en/huhailiang.html?lang=en
Proofread ByAdrian Cremin, Yingying XIA
Photo By