Researchers at the Southern University of Science and Technology (SUSTech) have elucidated a protein degradation pathway that ensures insulin is only secreted under a high glycemic state, thereby preventing hypoglycemia and hyperinsulinemia. Moreover, the pathway becomes dysregulated in metabolic diseases, and its suppression holds the potential to prevent obesity and diabetes.
Recently, Associate Professor Feng Rao’s research group from the School of Life Sciences at SUSTech published a paper in Nature Communications entitled “IP6-assisted CSN-COP1 competition regulates a CRL4-ETV5 proteolytic checkpoint to safeguard glucose-induced insulin secretion.” Last year, the same group discovered IP6 as an intermolecular glue between the Cullin RING E3 Ligases (CRL) and their inactivator CSN (PNAS, 2012). The new publication continues the group’s exploration at the frontier of CRL E3 ligase regulation.
The secretion of insulin, the primary hormone to clear blood glucose, is tightly regulated. Low insulin secretion leads to insufficient glucose clearance and diabetes. On the other hand, constitutively high insulin secretion will lead to a disease state known as hyperinsulinemia, which will be followed by insulin resistance and eventually diabetes. The pathways whereby glucose stimulates insulin secretion is well understood, however, comparatively less is known about how β-cell, the insulin-secreting cells, restrict unwanted or untimely insulin production and secretion.
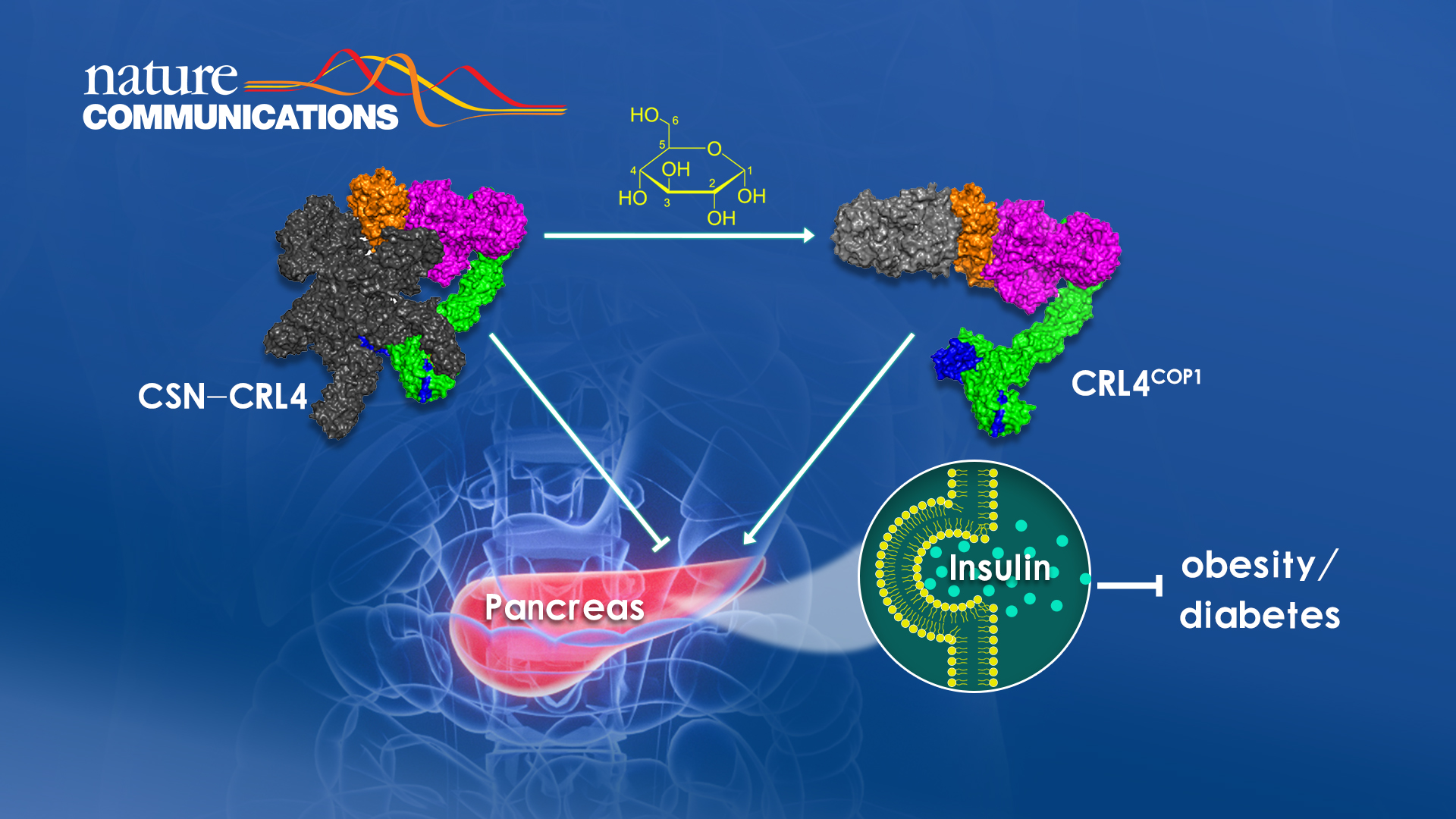
This paper published by Professor Rao Feng’s group discovered a glucose-responsive protein degradation axis that promotes insulin synthesis and secretion and is inhibited under hypoglycemia (Figure 1). Building on their earlier work identifying hexakisphosphate (IP6) as an essential metabolite bridging CRL4 and CSN together to inactivate CRL4, their research generated CSN mutant mice that disrupt the bridging function of IP6, and found that CRL4 is now activated and degrades ETV5, a protein linked to obesity and diabetes based on human genetics studies.

Figure 1. Schematic diagram illustrating that glucose activates CRL4 by removing the inactivator CSN. (A) The stimulatory effect of glucose eventually leads to insulin over-secretion, hyperinsulinemia, insulin resistance, obesity, and diabetes (B).
The team confirmed that ETV5 suppresses insulin production and secretion, thereby completing a IP6-CSN-CRL4-ETV5 pathway regulating blood glucose levels. Interestingly, the same pathway is feedback regulated by glucose, which activates CRL4 by dislodging IP6-CSN. Therefore, the CSN2 mutant mice generated by the researchers mimics constitutive glucose stimulation, with the long-term consequences being hyperinsulinemia, insulin resistance, and obesity. Finally, using a currently available drug, the group demonstrated that suppressing the CRL4-ETV5 proteolytic axis restores normal insulin and glucose levels in mice models that are prone to diabetes development.
The research explains how insulin hypersecretion, in the long run, can be damaging, leading to insulin resistance, obesity, and diabetes, which are severe health risks that would affect a large population worldwide. The work further provides proof-of-principle that targeting the CRL4-ETV5 axis ameliorates obesity and diabetes progression and is of therapeutic potential.
This work was primarily conducted in the laboratory of Associate Professor Feng Rao, who is also the corresponding author. The four co-first authors are research scholar Lin Hong and graduate students Yan Yuan and Luo Yifan from Professor’s Rao laboratory, and postdoctoral researcher Wing Yan So from the laboratory of Professor Han Weiping, based in Singapore Bioimaging Consortium.
Additional collaborations came from Dr. Jiang Nan from the Second Affiliated Hospital of SUSTech, Dr. Wang Fengchao from the National Institute of Biological Sciences, Beijing (NIBS), and Professor Billy Chow Kwok Chong from the University of Hong Kong (HKU).
This research was supported by the National Science Foundation of China (NSFC), the Shenzhen Municipal Science and Technology Innovation Commission, the Guangdong Provincial Department of Science & Technology, the Ministry of Science and Technology (MOST), the NSFC-Guangdong Joint Fund, and the Hong Kong Government GRF.
Article link: https://www.nature.com/articles/s41467-021-22941-3
Previous research article (PNAS, 2012): https://www.pnas.org/content/117/8/4117.short
Professor Feng Rao’s webpage: https://faculty.sustech.edu.cn/raof/en/
Proofread ByAdrian Cremin, Yingying XIA
Photo By