Recently, Associate Professor Wei Shu’s research group from the Department of Chemistry at the Southern University of Science and Technology (SUSTech) has achieved a series of progress in precise green catalysis, emphasizing first-row transition-metal catalyzed selective synthesis. Their relevant results have been published in some of the top academic journals, such as Angewandte Chemie, Nature Communications, and ACS Catalysis.

α-Chiral amides are widely present in biologically active molecules such as polypeptides and serve as precursors for synthesizing various functional molecules. Therefore, it is essential and valuable to develop a new method for synthesizing α-chiral amides with high efficiency and high enantioselectivity. The research group designed and synthesized a new class of bisoxazoline ligands and successfully applied them to nickel-catalyzed asymmetric hydrocarbofunctionalization of enamides with organic halides (Figure 1). Their findings were published in Angewandte Chemie, entitled “Regio- and enantioselective Ni-catalyzed formal hydroalkylation, hydrobenzylation and hydropropargylation of acrylamides to α-tertiary amides.” Lou Shi, a postdoctoral fellow from the Department of Chemistry at SUSTech, is the first author of this paper.
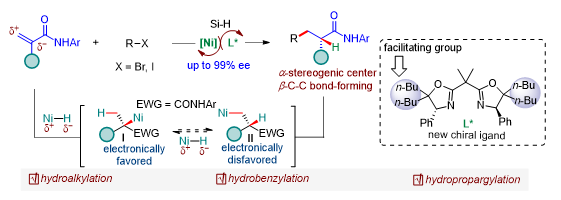
Figure 1. Nickel-catalyzed asymmetric hydrocarbofunctionalization of enamide
Chiral aliphatic amines and alcohols are widely found in natural products and drug molecules and serve as important intermediates in organic synthesis. Conventional methods are difficult to distinguish between two alkyl substituents with similar electrical properties and steric hindrance. Therefore, their enantioselective synthesis remains a formidable challenge. The research group developed a nickel-catalyzed asymmetric hydroalkylation of enamide or enol ester with alkyl iodide. Through the asymmetric construction of carbon-carbon bonds, chiral dialkyl amines and dialkyl alcohol derivatives were synthesized with high efficiency and high enantioselectivity (Figure 2). Their findings were published in Nature Communications, entitled “Enantioselective access to chiral aliphatic amines and alcohols via Ni-catalyzed hydroalkylations.” Shan Wang, a Ph.D. student from the Department of Chemistry at SUSTech, is the first author of this paper.
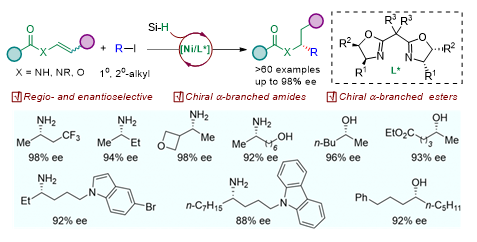
Figure 2. Nickel-catalyzed synthesis of dialkyl alcohols and amines
Multi-substituted olefins are important structural units, which are ubiquitous in natural products, bioactive molecules, and material molecules. Selective synthesis of multi-substituted olefins in a regio- and stereoselective fashion remains a challenge. The research group developed a nickel-catalyzed reductive cross-coupling of alkynes, alkyl halides, and α,β-unsaturated enones for the first time, affording multi-alkylated olefins by regio- and stereoselective dialkylation of alkynes (Figure 3). Their findings were published in Nature Communications, entitled “Ni-catalyzed regio- and stereodefined intermolecular cross-electrophile dialkylation of alkynes without directing group.” Yi-Zhou Zhan, a postdoctoral fellow from the Department of Chemistry at SUSTech, is the first author of this paper. This research was also highlighted by Professor Mark Lautens of the University of Toronto in Synfacts (Synfacts, 2021, 17, 0529).
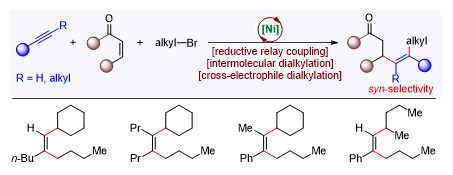
Figure 3. Nickel-catalyzed dialkylation of alkynes for the synthesis of multi-alkylated olefins
Selective transformation of unstrained, unactivated carbon-carbon bonds under mild conditions is a formidable challenge. The research group developed the γ-transarylation of amines by copper-catalyzed remote activation of inert carbon-carbon bond under mild conditions (Figure 4). This work provides a new avenue of utilizing unstrained, unactivated carbon-carbon bonds as a precursor for metal-catalyzed coupling reactions. Their findings were published in ACS Catalysis, entitled “Cu-catalyzed remote transarylation of amines via unstrained C-C functionalization.” Yu Wang, a postdoctoral fellow from the Department of Chemistry at SUSTech, is the first author of this paper.
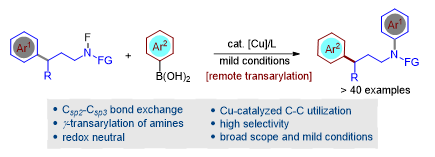
Figure 4. Cu-catalyzed remote transarylation of amines
The above-mentioned research was conducted at SUSTech, which serves as the sole address for contributing and corresponding author. Associate Professor Wei Shu is the corresponding author for all the research papers. This research was funded by the National Natural Science Foundation of China (NSFC), Guangdong Basic and Applied Basic Research Foundation, Guangdong Provincial Key Laboratory of Catalysis, the Shenzhen Municipal Science and Technology Innovation Commission, and the Shenzhen Nobel Prize Scientists Laboratory Project.
Links for the papers (In order of appearance above):
Angewandte Chemie: https://onlinelibrary.wiley.com/doi/10.1002/anie.202011339.
Nature Communications: https://www.nature.com/articles/s41467-021-22983-7.
Nature Communications: https://www.nature.com/articles/s41467-021-21083-w.
ACS Catalysis: https://pubs.acs.org/doi/10.1021/acscatal.0c04718.
Proofread ByAdrian Cremin, Yingying XIA
Photo By