Stoichiometry has a very important effect on the properties of oxides. Taking vanadium dioxide (VO2) as an example, the increase of oxygen vacancies significantly decreases the phase transition temperature from high-temperature metallic tetragonal R phase to low-temperature insulating monoclinic M1 phase. At the same time, excess oxygen can effectively stabilize two metastable insulating phases, the monoclinic M2 phase and the triclinic T phase. Up to date, there are still many difficulties in the fine regulation of oxygen content in oxides, which is a very important and challenging research topic.
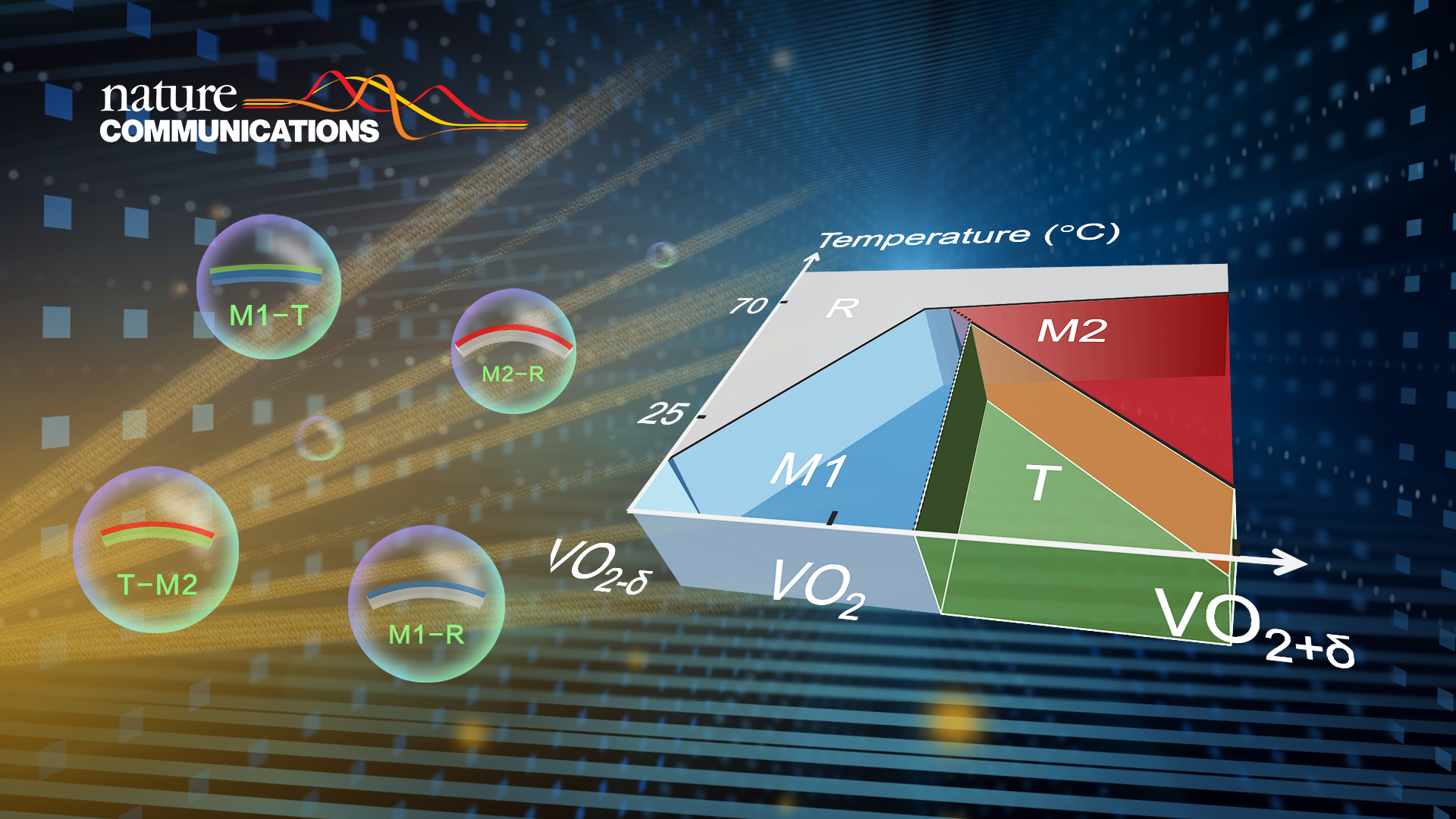
Recently, Professor Chun Cheng’s research group from the Department of Materials Science and Engineering at the Southern University of Science and Technology (SUSTech) developed a novel oxide inhibitor-assisted chemical vapor deposition (CVD) method to effectively regulate the oxygen content of VO2. This method stabilizes all the insulation phases (M1, M2, and T) in one-dimensional VO2 single crystals at room temperature and provides the empirical synthesis phase diagram. It offers experimental evidence to verify and revise the stoichiometry-temperature phase diagram of VO2.

Figure 1. (a) Classical stoichiometry-temperature phase diagram of VO2. (b) Raman spectra of different VO2 phases
Notably, the concept of a “phase transition route device” is proposed according to the phase diagram. A typical example of this concept, a family of single-crystalline VO2 actuators (M1-R, T-M2, M1-T, and M2-R), is proposed and realized by the laterally asymmetric stoichiometry engineering. These single-crystalline actuators have achieved the best actuation performance of VO2-based actuators. This research has been published online in Nature Communications, entitled “Phase management in single-crystalline vanadium dioxide beams.”
In oxide inhibitor-assisted growth, the reactive oxide precursor is mixed with inert oxides (such as silicon dioxide, aluminum oxide, etc.). The evaporation of active substances is limited for a good reaction kinetics control. As a result, the nucleation density, morphology, chemical composition, and other properties of final products are finely modulated. Chun Cheng’s group has used this method for the controlled fabrication of monolayer two-dimensional thin films such as MoS2 in ACS Nano, 2020, 14, 7593-7601.
In low-pressure CVD fabrication of VO2, the oxidation inhibitor (SiO2) plays an essential role in a complex way. When the temperature is below 850 °C, SiO2 effectively disperses the vanadium pentoxide (V2O5, reactive vanadium source) particles, preventing their aggregation and recrystallization. Therefore, the addition of SiO2 effectively enhances the evaporation and reduction of V2O5 at a relatively low temperature.
Once the reaction temperature reaches 850 ℃, SiO2 can absorb V2O5 and hinder its evaporation process. Most vanadium sources are fixed in the precursor powder in this stage, but oxygen produced by the reduction of V2O5 is released into the reaction system. Therefore, oxygen partial pressure in the reaction atmosphere can be effectively increased by adding SiO2. Consequently, SiO2 can effectively reduce the nucleation density of VO2 micro/nanowires and increase their size. More importantly, SiO2 increases the oxygen content and realizes the selective stabilization of different phase structures (Figure 2b).
The experimental results (Figure 2c) show that under different reaction conditions, the oxygen content in the product (corresponding to the ωO frequency in the Raman spectra) can be continuously regulated, demonstrating the powerful modulation capability of the reported oxide inhibitor-assisted CVD strategy. It is worth noting that the VO2 beams can produce an axial strain of ~1.65% across the M2-R transition (Figure 2g), demonstrating the good phase uniformity of the beam and its great potential in high-performance actuation devices.
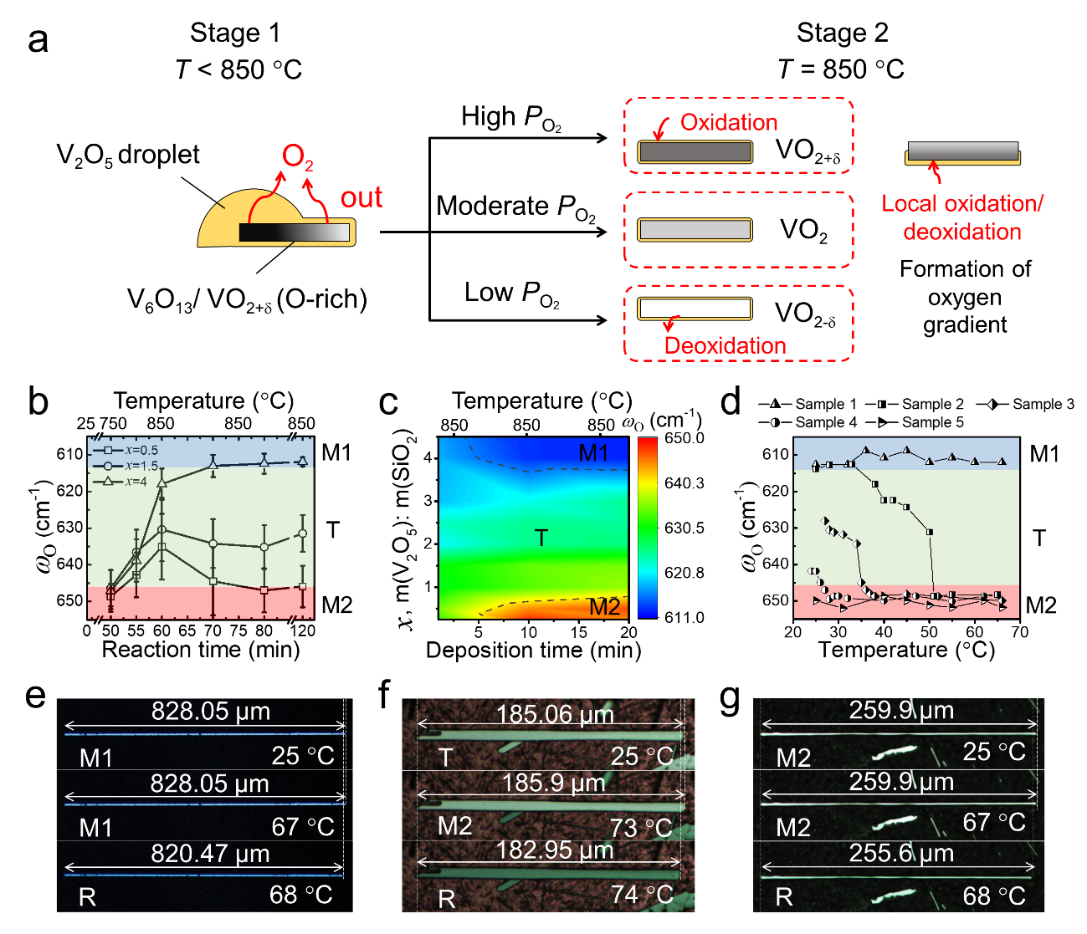
Figure 2. (a) Schematics for oxide inhibitor-assisted CVD growth of VO2 beams. (b, c) Influence of SiO2 dosage and reaction conditions on the phase structure of VO2 (x is the mass ratio of V2O5 to SiO2). (d) Phase transition routes and (e-g) axial strain of VO2 beams with different room temperature phases.
Prof. Cheng’s group reported a novel single-crystalline VO2 actuator (SCVA) in 2019 (Advanced Functional Materials). Its working mechanism is described as follows: Due to the asymmetric distribution of wetting layer in the reaction process, as-grown tungsten-doped single-crystalline VO2 micro/nanobeams have a gradient distribution of oxygen defects in the radial direction so that the two sides of the micro/nanobeams have different M1-R phase transition temperatures.
When the two sides are occupied by the M1 and R phases, respectively, their strain difference will drive the bending of beams (Figure 3a). This bending device is the so-called M1-R type SCVA. By utilizing the asymmetric droplet distribution, the oxide inhibitor-assisted reaction system provides an oxygen-rich reaction atmosphere and leads to forming a radial oxygen gradient. As a result, the T and M2 phases, respectively, occupy the two sides of VO2 beams and drive their bending actuation. These beams are called T-M2 type SCVA. Notably, the working temperature and amplitude of T-M2 SCVAs can be directly affected by the amount of SiO2.
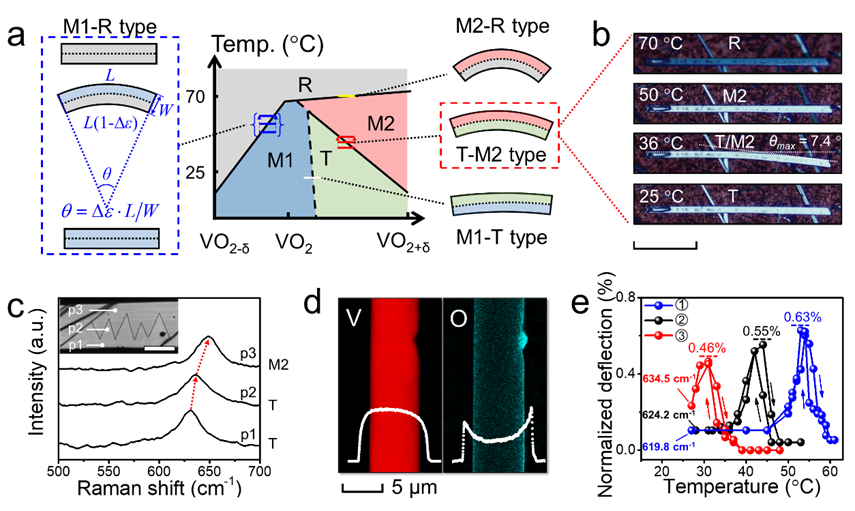
Figure 3. (a) Working mechanism of SCVAs. (b) Bidirectional bending actuation of a T-M2 SCVA upon the change of temperature. (c) Domain structure of a bending T-M2 SCVA. (d) Element distribution along the SCVA. (e) Temperature-dependent deflection curve of T-M2 SCVAs with different oxygen contents.
However, because the phase boundary between the M2 and R phases is too flat, the small oxygen gradient cannot realize the high-performance M2-R SCVA. To solve this problem, tungsten dioxide and SiO2 are mixed with V2O5 to widen the oxygen gradient effectively. By this means, M2-R SCVAs has been successfully produced, and they demonstrate the expected complex domain evolution process (Figure 4a-c).
By using the large axial strain of the M2-R transition, M2-R SCVAs show superior actuation performance to most existing actuation devices and conventional VO2 actuators in terms of work density and work speed, indicating that the M2-SCVA has reached the theoretical performance limit of VO2-based actuators. Additionally, the SCVA also demonstrates excellent stability in various operating environments due to its minimalist device architecture.
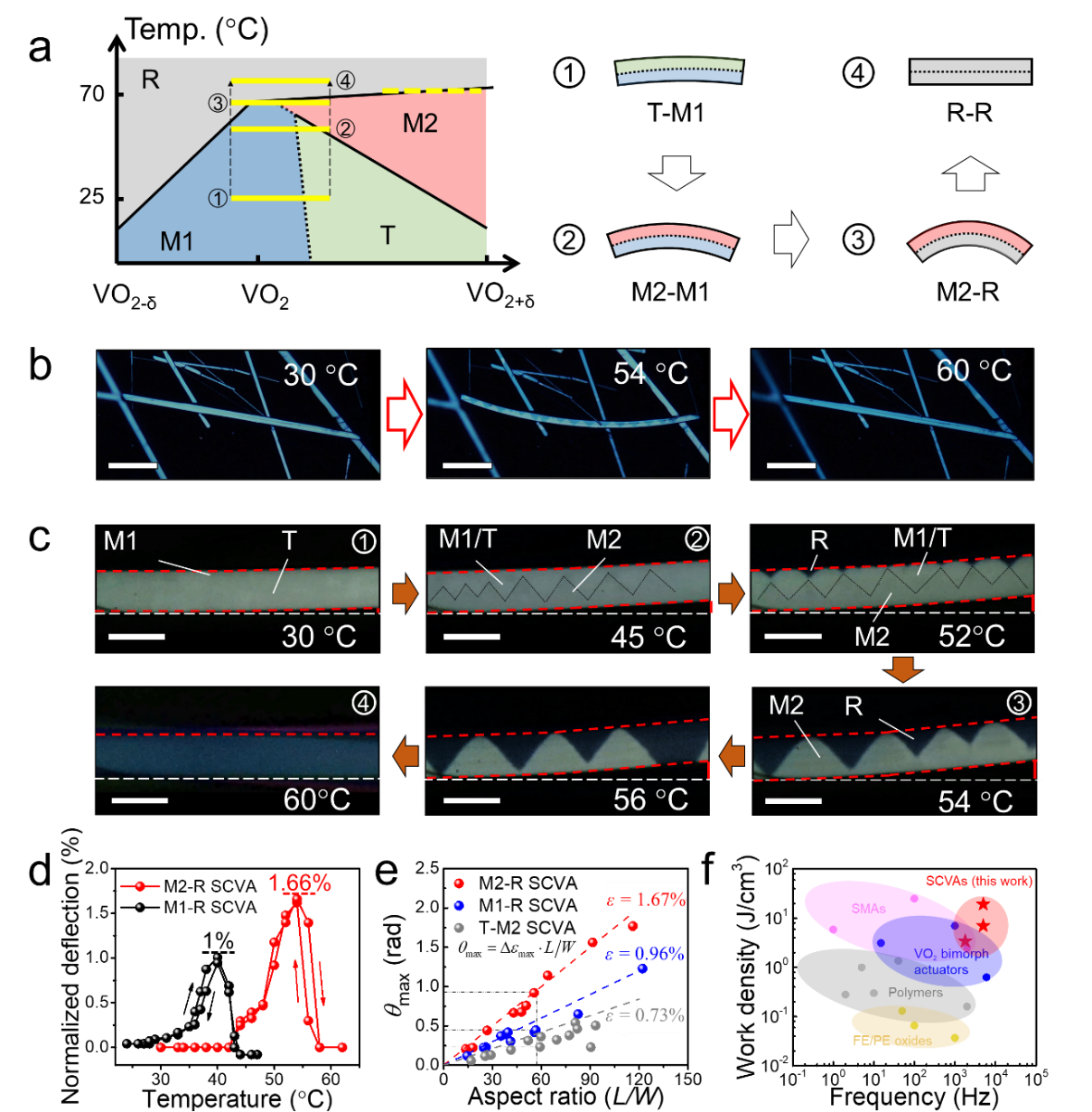
Figure 4. (a) Working mechanism of M2-RSCVA. (b) Bidirectional bending actuation and (c) domain evolution process of an M2-R SCVA upon temperature change. (d) Temperature-dependent deflection curves of an M2-R SCVA and an M1-R SCVA. (e) The maximum deflection angle of different SCVAs with various aspect ratios. (f) Work density and work speed of SCVAs compared with other actuators.
Video 1. Silent forest in action -VO2 SCVAs upon heating.
This work not only provides sufficient sample materials for the study of phase transition mechanism and characteristics of VO2 but also provides some new inspirations for the stoichiometry engineering of other oxide systems. In addition, this study demonstrates that the controlled phase transition route and ordered domain structure play a decisive role in the performance improvement of VO2 devices, which may trigger future advances in the applications of other phase change materials.
This work was financially supported by the National Natural Science Foundation of China (NSFC), Basic Research Project of Science and Technology Plan of Shenzhen, and the Foundation of Shenzhen Science and Technology Innovation Committee. This work was also supported by the SUSTech Core Research Facilities and Professor Ning Wang’s group from the Department of Physics at the Hong Kong University of Science and Technology (HKUST).
The first author of this paper is Mr. Run Shi, a Ph.D. student in the collaborative program between SUSTech and HKUST. Professor Chun Cheng is the sole correspondent of the work, and SUSTech is the first communication unit. Prof. Cheng’s research group has been focusing on the preparation and applications of VO2 for many years and welcomes any cooperation with people interested in this smart material.
Original paper:
Nature Communications: https://www.nature.com/articles/s41467-021-24527-5
Related paper information and links:
Advanced Functional Materials: https://onlinelibrary.wiley.com/doi/10.1002/adfm.201900527
ACS Nano: https://pubs.acs.org/doi/10.1021/acsnano.0c03469
Applied Physics Letters: https://aip.scitation.org/doi/10.1063/5.0020597
Applied Physics Review: https://aip.scitation.org/doi/abs/10.1063/1.5087864
Applied Physics Review: https://aip.scitation.org/doi/10.1063/1.5124672
Advanced Materials Interfaces: https://doi.org/10.1002/admi.201901582
Journal of Physical Chemistry C: https://pubs.acs.org/doi/abs/10.1021/acs.jpcc.7b08946
Advanced Intelligent Systems: https://onlinelibrary.wiley.com/doi/10.1002/aisy.202000051
Journal of Materials Chemistry A: https://doi.org/10.1039/C8TA11071A
Scientific Reports: https://www.nature.com/articles/s41598-018-29652-8
ACS Applied Electronic Materials: https://pubs.acs.org/doi/10.1021/acsaelm.1c00260
Advanced Optical Materials: https://www.onlinelibrary.wiley.com/doi/full/10.1002/adom.201400483
Additional links:
Webpage of Prof. Chun Cheng’s group: https://faculty.sustech.edu.cn/chengc/
Webpage for recruitment: https://faculty.sustech.edu.cn/?cat=11&tagid=chengc&orderby=date&iscss=1&snapid=1
Proofread ByAdrian Cremin, Yingying XIA
Photo By