Recently, Professor Zhouguang Lu from the Department of Materials Science and Engineering (MSE) at the Southern University of Science and Technology (SUSTech) reported a simple yet straightforward strategy to improve the cycling stability of ultra-high voltage LiCoO2 at 4.6V. This study, entitled “Dextran sulfate lithium as versatile binder to stabilize high-voltage LiCoO2 to 4.6 V,” was published in Advanced Energy Materials, a high-profile journal in material science.
With the commercialization of 5G technology, the demand for lithium-ion batteries (LIBs) with high capacity is ever-growing. In the portable electronic market, LiCoO2 still dominates the cathode materials for LIBs due to its exceptionally high volumetric energy density. LiCoO2 has a theoretical capacity of 274 mAh g-1. However, the currently approachable capacity of LiCoO2 is limited to 175 mAh g-1 by a cut-off charge voltage of 4.45 V.
Further increasing the attainable capacity of LiCoO2 requires extracting more lithium ions by elevating the charging voltage. By a cut-off voltage of 4.6 V, the capacity could increase to 220 mAh g-1, but it will unavoidably bring about structure and surface instability issues that deteriorate the cyclability, including deteriorative cathode electrolyte interphase formation, O2 loss, and Co ions dissolution. Thus, increasing the charging voltage to 4.6 V is still a big challenge for high-performance LiCoO2. However, the commonly used element doping and surface coating methods involve complex processes at a high cost, depressing their industrial utilization. Therefore, it is urgent to develop a simple strategy to improve the cycling stability of high-voltage LiCoO2.
Professor Lu and his team proposed a novel approach to stabilize the cycling of LiCoO2 to 4.6 V by replacing the conventional polyvinylidene fluoride (PVDF) binder with dextran sulfate lithium (DSL). As a result, a coulombic efficiency of almost 100% and 93.4% capacity retention after 100 cycles has been achieved. The aqueous DSL binder could be evenly coated onto the surfaces of LiCoO2 particles to function as an artificial interface, significantly improving the surficial and structural stability of LiCoO2 under high voltage, as shown in Scheme 1.
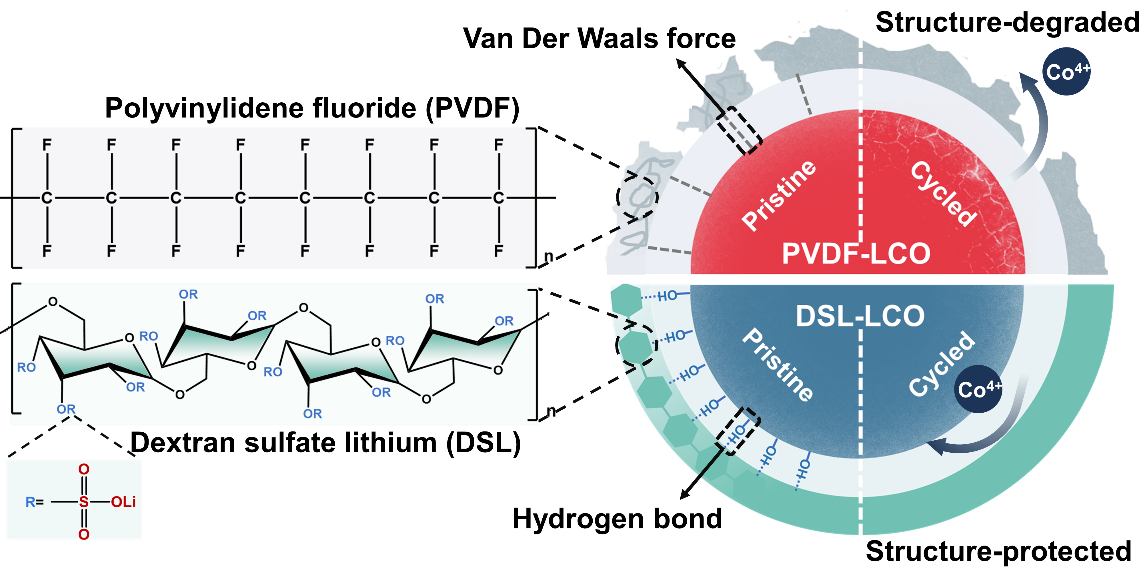
Scheme 1. Schematic illustration of the distinctive Van der Waals and hydrogen bonding forces from PVDF and DSL binder, respectively, in affecting the cycling stability of LiCoO2.
To further understand how the DSL binder influences the structural evolution behavior of LiCoO2, in-situ XRD measurements of PVDF-LCO and DSL-LCO cathodes were performed, as shown in Figure 1. The (003) peak shifting in the DSL-LCO is much weaker than the PVDF-LCO, illustrating that the uniform DSL coating layer significantly suppresses the harmful phase transition of LiCoO2 from the O3 phase to the H1-3 phase near 4.55 V.
In addition, the focused ion beam (FIB) was performed in Figure 1(d). It found that the PVDF-LCO particle displays interior degradation with obvious microcrack, while the DSL-LCO particle maintains superior cross-section morphology. Clearly, the uniform coating of the DSL binder greatly enhances the structural stability of the LiCoO2 under high voltage.
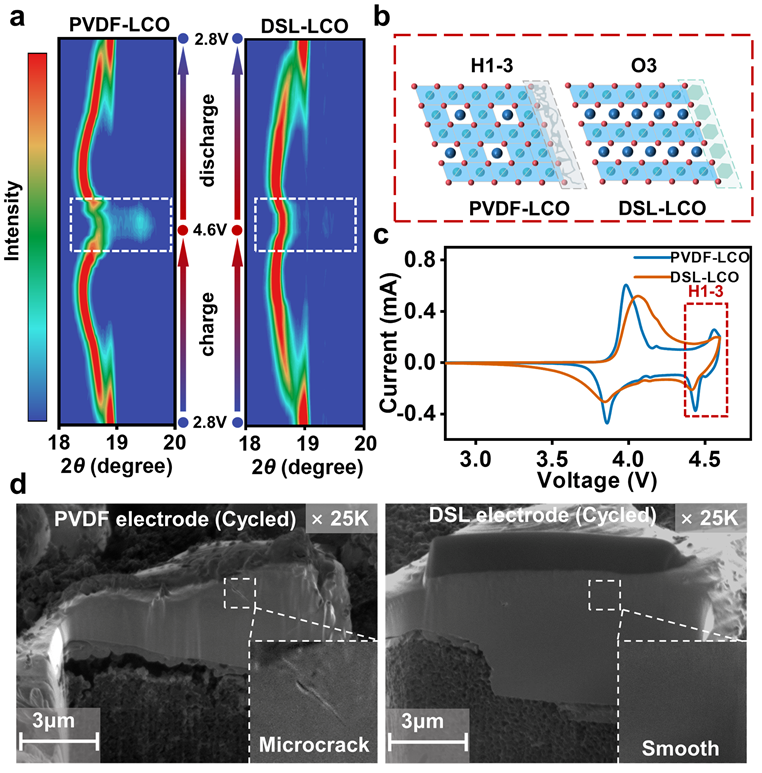
Figure 1. a) In-situ X-ray diffraction (XRD) evolution of PVDF-LCO and DSL-LCO samples at the (003) diffraction peak during the first cycle. b) Schematic illustration of the atomic stacking for PVDF-LCO and DSL-LCO at high voltage. c) The cyclic voltammetry results of PVDF-LCO and DSL-LCO electrodes. d) Focused ion beam-scanning electron microscopy (FIB-SEM) images of PVDF-LCO and DSL-LCO after 50 cycles.
The surface microstructure of PVDF-LCO and DSL-LCO cycled samples were also analyzed by the high-resolution transmission electron microscopy (HRTEM), as shown in Figure 2. Relatively slight surface erosion and structural collapse with uniform DSL-coated layer were observed in the DSL-LCO particle, in contrast with the severe degradation of the surface structure of PVDF-LCO particle.

Figure 2. High-resolution transmission electron microscopy (HRTEM) and fast Fourier transform (FFT) images of the electrode powders after 50 cycles: a) PVDF-LCO. b) DSL-LCO.
To further investigate how the DSL binder functioned on stabilizing the high-voltage LiCoO2, in-situ Raman spectroscopy characterizations were conducted. Figure 3(a) shows the variation of O-Co-O bending (Eg) and Co-O stretching (A1g) of LiCoO2, respectively. PVDF-LCO electrode exhibited dramatic attenuation in the Eg and A1g peaks, while DSL-LCO cathode showed superior reversibility of Eg and A1g peaks after cycling.
Further research, with the help of soft X-ray absorption spectroscopy (XAS) measurements, was performed to corroborate the stability of Co-O bonds. As shown in figure 3(c), Co4+(a1g)-O 2p and Co4+(eg)-O 2p peaks emerged with the increase of cycle number in the PVDF-LCO electrode. In contrast, DSL-LCO cathode showed less accumulation of Co4+ at this TFY probing depth, which could be attributed to the aforementioned interaction between DSL binder and surficial Co-O bonds, further alleviating the deep degradation in the LiCoO2 structure.

Figure 3. a) In-situ Raman spectra evolution of the PVDF-LCO and DSL-LCO samples at the Eg and A1g Raman active vibrational peaks during the first cycle. b) The selected Raman spectra at different states of charge and discharge. c) O K-edge XAS spectra of PVDF-LCO and DSL-LCO with TFY detection for the 1st, 50th, and 200th cycles.
As shown in Figure 4, the electrochemical impedance spectra (EIS) test and X-ray photoelectron spectroscopy (XPS) were performed to explore the interfacial properties between the LiCoO2 and electrolyte. It found that the PVDF-LCO sample markedly increased in the resistance, electrolyte decomposition, and Co dissolution, while the DSL-LCO sample exhibited significant differences with promising interfacial performance. This could be attributed to the uniform DSL coating layer alleviating the electrolyte decomposition and suppresses the Co dissolution, further contributing to the superior cycle performance of the DSL-LCO battery.
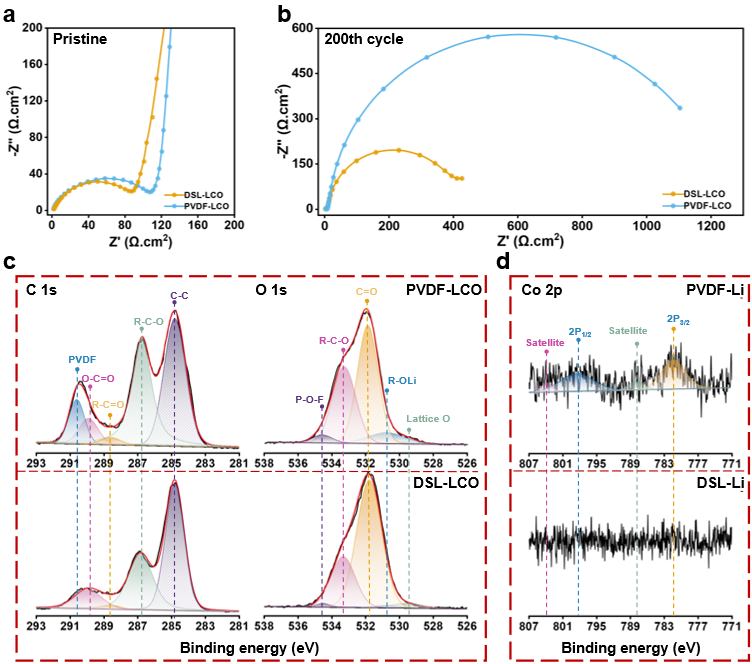
Figure 4. (a & b) Electrochemical impedance spectroscopy of a) PVDF-LCO and b) DSL-LCO after 200 cycles at 1C. (c & d) X-ray photoelectron spectroscopy (XPS) of c) C 1s and O 1s XPS spectra of PVDF-LCO and DSL-LCO cathodes after 200 cycles and d) Co 2p XPS spectra of PVDF-Li and DSL-Li lithium metal anodes after 200 cycles.
This work was led by SUSTech. He Huang, a master’s student from SUSTech, is the first author of this study. Zhiqiang Li, a Ph.D. student from SUSTech, and Shuai Gu, a visiting student from SUSTech, are the major contributors to this paper research. Professor Zhouguang Lu from SUSTech is the corresponding author.
This work was financially supported by the Basic Research Project of the Science and Technology Innovation Commission of Shenzhen, Shenzhen Key Laboratory of Interfacial Science and Engineering of Materials, the Leading Talents of Guangdong Province Program, Guangdong-Hong Kong-Macao Joint Laboratory, and the National Natural Science Foundation of China (NSFC).
Assistance on SEM, TEM, ICP-MS, and XRD characterizations was received from the SUSTech Core Research Facilities (SCRF) and DFT simulations from the Center for Computational Science and Engineering at SUSTech. The XAFS experiments were conducted in the BL02U Beamlines at the Shanghai Synchrotron Radiation Facility (SSRF).
Paper link: https://doi.org/10.1002/aenm.202101864
Proofread ByAdrian Cremin, Yingying XIA
Photo By