Visible light is an ultimately green energy source, so it is crucial to develop efficient chemical transformation using visible light catalysis. Recently, Associate Professor Wei Shu’s group at the Southern University of Science and Technology (SUSTech) has made a series of developments in the field of visible-light catalyzed selective transformations. Their results have been published in high-level journals, such as Angewandte Chemie and ACS Catalysis.
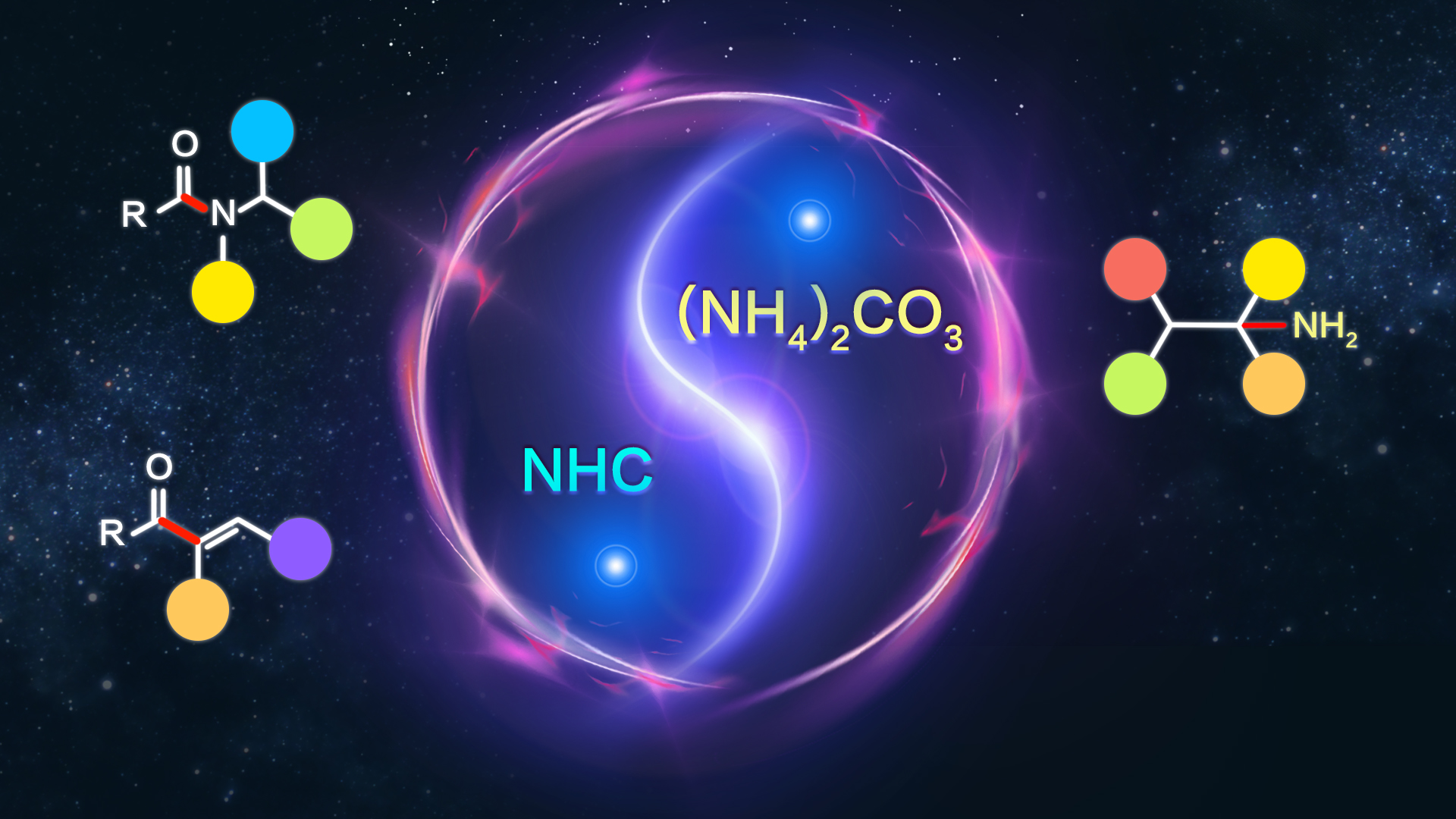
Aliphatic primary amines are widely found in pharmaceutical, pesticide, and material molecules. They serve as precursors for secondary and tertiary amines, as well as other functional groups. Thus, efficient and selective synthesis of primary amines is highly desirable.
Traditional methods to access primary amines often require multiple reaction steps, harsh reaction conditions, and/or the use of advanced precursors. They often suffer from the challenge of over-alkylation to afford a mixture of primary, secondary, and tertiary amines. In addition, steric congested primary amines, such as α-tertiary primary amines, are difficult to access. Therefore, developing highly selective and general methods for synthesizing primary amines from simple and readily available materials is a long-term challenge.
Prof. Wei Shu’s group developed an unprecedented synthesis of primary amines from alkenes via metal-free regioselective hydroamination at room temperature (Scheme 1). Inorganic ammonium salt was used as an ammonia surrogate for the first time. This allowed for efficient conversion of terminal and internal alkenes into linear, α-branched, and α-tertiary primary amines under mild conditions. This method provides a straightforward and powerful approach to a broad spectrum of advanced, highly functionalized primary amines, particularly in pharmaceutical chemistry and other areas.
This work was published in Angewandte Chemie, entitled “Direct access to primary amines from alkenes by alkenes by selective metal-free hydroamination” and was selected as a hot paper. Dr. Yi-Dan Du from the Department of Chemistry at SUSTech is the first author of this work.
This work has received great attention from academia and industry. Professor Benjamin List at the Max-Planck Institute in Germany highlighted this work in Synfacts. Chemists and engineers from pharmaceutical companies highlighted this work in Organic Process Research & Development. Professor Peng-Fei Li at Xi’an Jiaotong University highlighted this work in the Chinese Journal of Organic Chemistry.

Scheme 1. Visible-light catalyzed direct access to primary amines from ammoniums and alkenes
Amide is an extremely important class of molecules in natural products, pharmaceutical molecules, organic materials, and life sciences. Using readily available and cost-effective precursors for straightforward amide synthesis with atom economy under mild conditions is attractive yet challenging.
Recently, Prof. Shu’s group reported the direct synthesis of amides from aldehydes and imines in a 100% atom-economic manner (Scheme 2). The redox-neutral C-N bond-forming process was enabled by the dual catalysis of visible-light and nitrogen heterocyclic carbene (NHC) at room temperature. This protocol features the unprecedented umpolung of imines to generate N-centered radicals.
The work was published in ACS Catalysis, entitled “Catalytic, metal-free amide synthesis from aldehydes and imines enabled by a dual-catalyzed umpolung strategy under redox-neutral-neutral-conditions”. Dr. Ming-Shang Liu from the Department of Chemistry at SUSTech is the first author of this work.
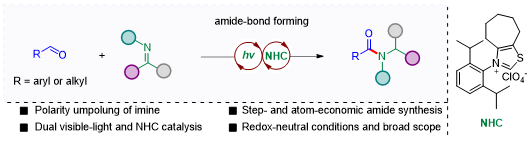
Scheme 2. Visible-light and NHC-catalyzed amide synthesis from imines and aldehydes
Carbon-carbon bonds are one of the most essential chemical bonds in organic compounds. Therefore, direct functionalizations of two distinct inert C-H bonds represent the most ideal way to construct C-C bonds. Recently, Prof. Shu’s team reported an intermolecular vinylation of aldehydes using alkenes as the vinylating reagents through sequential two-fold C-H functionalizations (Scheme 3).
The merging of visible light and NHC-catalysis allows for the coupling of alkenes with aldehydes through a dual catalysis relay enabled cross-dehydrogenative coupling mechanism. The use of diphenoquinone is essential for the success of this reaction, which plays an intriguing two-fold role in the reaction, as an electron acceptor and a radical reservoir for the radical coupling enabling the C-C forming process.
The results were recently published in ACS Catalysis, entitled “Dual catalysis relay: Coupling of aldehydes and alkenes enabled by visible-light and NHC-catalyzed cross-double C-H functionalizations”. Dr. Ming-Shang Liu from the Department of Chemistry at SUSTech is the first author of this work.
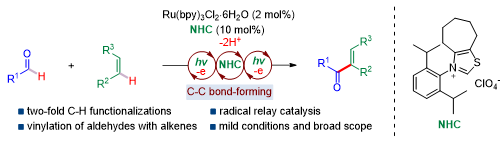
Scheme 3. Visible-light and NHC-catalyzed two-fold C-H cross-couplings
The above-mentioned research was conducted at SUSTech, which serves as the sole contributing and corresponding author. Associate Professor Wei Shu is the corresponding author for all the research work.
This work was supported by the National Natural Science Foundation of China (NSFC), the Guangdong Natural Science Foundation, the Key Laboratory of Catalytic Chemistry of Guangdong Province, the Peacock Project of Shenzhen City, the Science and Technology Commission of Shenzhen, the Shenzhen Nobel Prize Laboratory, and the SUSTech Core Research Facilities.
Paper links (In order of appearance above):
Angewandte Chemie: https://onlinelibrary.wiley.com/doi/10.1002/anie.202016679
ACS Catalysis: https://pubs.acs.org/doi/10.1021/acscatal.0c04070
ACS Catalysis: https://pubs.acs.org/doi/10.1021/acscatal.1c02890
Related links:
Highlight in Synfacts: https://www.thieme-connect.de/products/ejournals/pdf/10.1055/s-0040-1706173.pdf?update=true&update=true
Highlight in Organic Process Research & Development: https://pubs.acs.org/doi/10.1021/acs.oprd.1c00095
Highlight in Chinese Journal of Organic Chemistry: http://sioc-journal.cn/Jwk_yjhx/EN/10.6023/cjoc202100055
Homepage of Prof. Wei Shu’s group: https://faculty.sustech.edu.cn/shuw/en/
Proofread ByAdrian Cremin, Yingying XIA
Photo By