As an important main group element in organic chemistry, Boron plays a pivotal role in modern organic synthetic chemistry and usually adopts a trigonal planar configuration with an empty p-orbital. In the presence of amines, phosphines, N-heterocyclic carbenes (NHCs), etc., the empty p-orbitals of tricoordinate boron compounds are apt to accept the electrons from these Lewis bases or nucleophiles, leading to tetracoordinate boron compounds.
Importantly, tetracoordinate boron compounds are a fundamental class of molecules, which are the key intermediates in many organoboron-related chemical transformations. They also promise new kinds of light-emitting materials.
Associate Professor Chuan He’s team from the Department of Chemistry at the Southern University of Science and Technology (SUSTech) has published a breakthrough paper in the Journal of the American Chemical Society, one of the most impactful and prestigious journals in chemistry.
Their research was entitled “Catalytic Enantioselective Construction of Chiroptical Boron-Stereogenic Compounds.” They looked at methods to access enantioenriched boron-stereogenic compounds through an asymmetric copper-catalyzed azide−alkyne cycloaddition (CuAAC) reaction.
The first catalytic enantioselective constructing the boron-stereogenic compounds
Although chiral organoboron compounds (mainly featuring carbon-stereogenic groups attached to the boron atom) are very useful reagents in asymmetric synthesis, rare examples possess tetrahedral boron-stereogenic centers.
Methods to access enantioenriched boron-stereogenic compounds are restricted to resolution or a stoichiometric chiral substrate-induced diastereoselective approach. To date, catalytic asymmetric synthesis of enantioenriched boron-stereogenic compounds has remained unknown.
With the continued interest in synthesizing heteroatom-stereogenic compounds, Prof. He’s group reported the first catalytic enantioselective construction of boron-stereogenic compounds via a CuAAC reaction.
The tetracoordinate boron scaffold bearing configurationally locked N,N ligands caught their attention due to its rigidity and good photoelectric properties. After optimizing the conditions, the group next examined the reaction scope.
Various electron-donating and -withdrawing functional groups were incorporated in good yields with high enantioselectivities to form the desired boron-stereogenic products. The team then carried out the racemization experiment of selected products with a different functional group. The results show that the electron-withdrawing group on the pyrrole ring or the electron-donating group on the pyridine ring can lead to a stronger interaction with the coordination covalent B1–N1 bond.
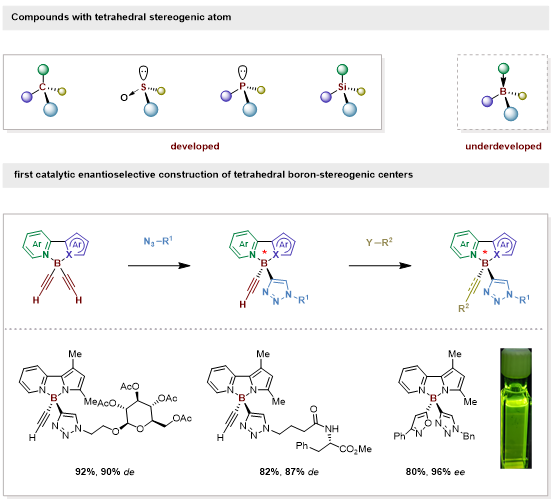
Figure 1. Constructing the boron-stereogenic compounds via CuAAC reaction
It is worth mentioning that these novel boron-stereogenic compounds show good optical properties that could have potential applications as chiral fluorescent probes or optoelectronic materials. This work not only provides a new idea for the synthesis of boron-stereogenic compounds but also opens up a new direction of chiral boron chemistry.
Bing Zu, a joint doctoral candidate supported by the joint doctoral program between SUSTech and Harbin Institute of Technology (HIT), and Yonghong Guo, a doctoral candidate from SUSTech, are the co-first authors of this paper. Associate Professor Chuan He is the sole corresponding author, while SUSTech is the sole communication unit.
This work was supported by the National Natural Science Foundation of China (NSFC), Guangdong Provincial Key Laboratory of Catalysis, Shenzhen Science and Technology Innovation Committee, and the Stable Support Plan Program of Shenzhen Natural Science Fund.
Paper link: https://doi.org/10.1021/jacs.1c08482
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo By