Associate Professor Ji Liu from the Department of Mechanical and Energy Engineering at the Southern University of Science and Technology (SUSTech) recently reported the applications of functional hydrogel bioadhesives in wound closure, tissue repair, and bioelectronic interfaces. Based on these results, two important papers have been published in Advanced Functional Materials, a high-profile journal in material science.
Recent flexible electronics technology development has provided unprecedented opportunities to enable implantable bioelectronics for long-term disease monitoring and treatment and are expected to integrate the human body and machine. Sutures and staples have been routinely used for bioelectronics implantation and fixation. However, they are associated with severe tissue damage and biological issues such as inflammation response. Moreover, this kind of implantation is not suitable for dynamic or soft and fragile tissues (such as the heart, blood vessels, and nerves), which presents great challenges for the implantation of electronic devices. Hydrogels have emerged as one of the most promising material candidates to seamlessly integrate humans and electronics, in light of biological, mechanical, chemical, and physical similarity to tissues.
Hydrogel bioadhesion technology has offered unprecedented progress in minimally invasive surgeries, which are routinely performed to reduce postoperative complications, recovery time, and patient discomforts. However, they are challenged by the construction of bioelectronic interfaces. For example, commercially-available hydrogel adhesives (such as Fibrin glue) exhibit slow adhesion formation (over a few minutes) and weak adhesion (interfacial toughness below 20 J m-2). Synthetic hydrogel adhesives could adhere to tissues, but they are challenged by either non-biodegradability or small molecules residues, accompanied by undesirable immunological side effects during in vivo applications.
Based on this, Prof. Liu’s team developed a kind of synthetic hydrogel adhesives, featuring instant and robust tissue adhesion, superior biocompatibility, inflammation-free, excellent biodegradability, and allowance for wet and highly dynamic environments. In this work, a design and fabrication strategy was delivered to fabricate tissue bioadhesives from pure polymer precursors without involving any monomers. Therefore, all those biological issues (i.e., cytotoxicity, immunological response, etc.) associated with monomer residues could be avoided entirely.
All the in vivo results demonstrated that these hydrogel adhesives don’t induce any inflammatory response during the wound sealing in both liver and blood vessels, promising for immediate repairing of vascular defects and surgical.
Kuan Zhang, a former research assistant in Prof. Liu’s team, Xingmei Chen and Yu Xue, Ph.D. candidates from SUSTech, are the co-first authors of this paper. Prof. Ji Liu is the corresponding author.
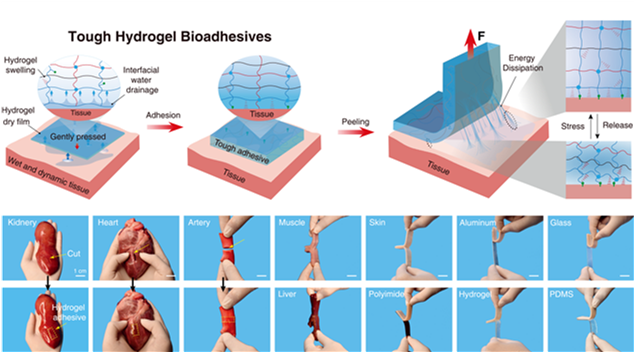
Figure 1. Hydrogel bioadhesives form a robust interface with various biological tissues and engineering substrates.
Current tissue-electronics interfaces are predominately constructed through either weak Van der Waals interaction, strong chemical bonding, or both. However, delicate biological tissues (e.g., blood vessels and nerves) might be severely destroyed when these bioelectronics are removed, resulting in drastic pain or even massive bleeding. Despite recent reports on trigger-detachable hydrogel bioadhesion, these strategies may be challenged by either complicated molecule design and synthesis, non-biocompatible triggers, long waiting time, limited variation in interfacial toughness before and after triggering, or complicated geometry design of the adhesive layer, consequently hampering their practical utility.
Therefore, the development of hydrogel-based bioadhesives with highly controllable bonding interfaces to construct stable and efficient bioelectronic interfaces can provide strong support for the development of emerging bioelectronic devices and personalized medical devices. Based on this, Prof. Liu’s team elaborately introduced dynamic interactions (i.e., boronate-diol complexation) as energy dissipation components, leading to an interfacial toughness over 400 J m−2. By dissociating the boronate-diol complexes with a small yet competitive molecule (i.e., glucose), the work exhibited a faster response to external stimuli (within 3 minutes). The overall interfacial toughness decreases more than 10-times, allowing for benign detachment without any damage.
Yu Xue, a Ph.D. candidate from SUSTech, Jun Zhang, a postdoctoral researcher at SUSTech, and Xingmei Chen, a Ph.D. candidate from SUSTech, are the co-first authors of this paper. Prof. Ji Liu is the corresponding author.

Figure 2. On-demand triggered detachment of hydrogel bioadhesives upon treatment with biocompatible molecules (i.e. glucose).
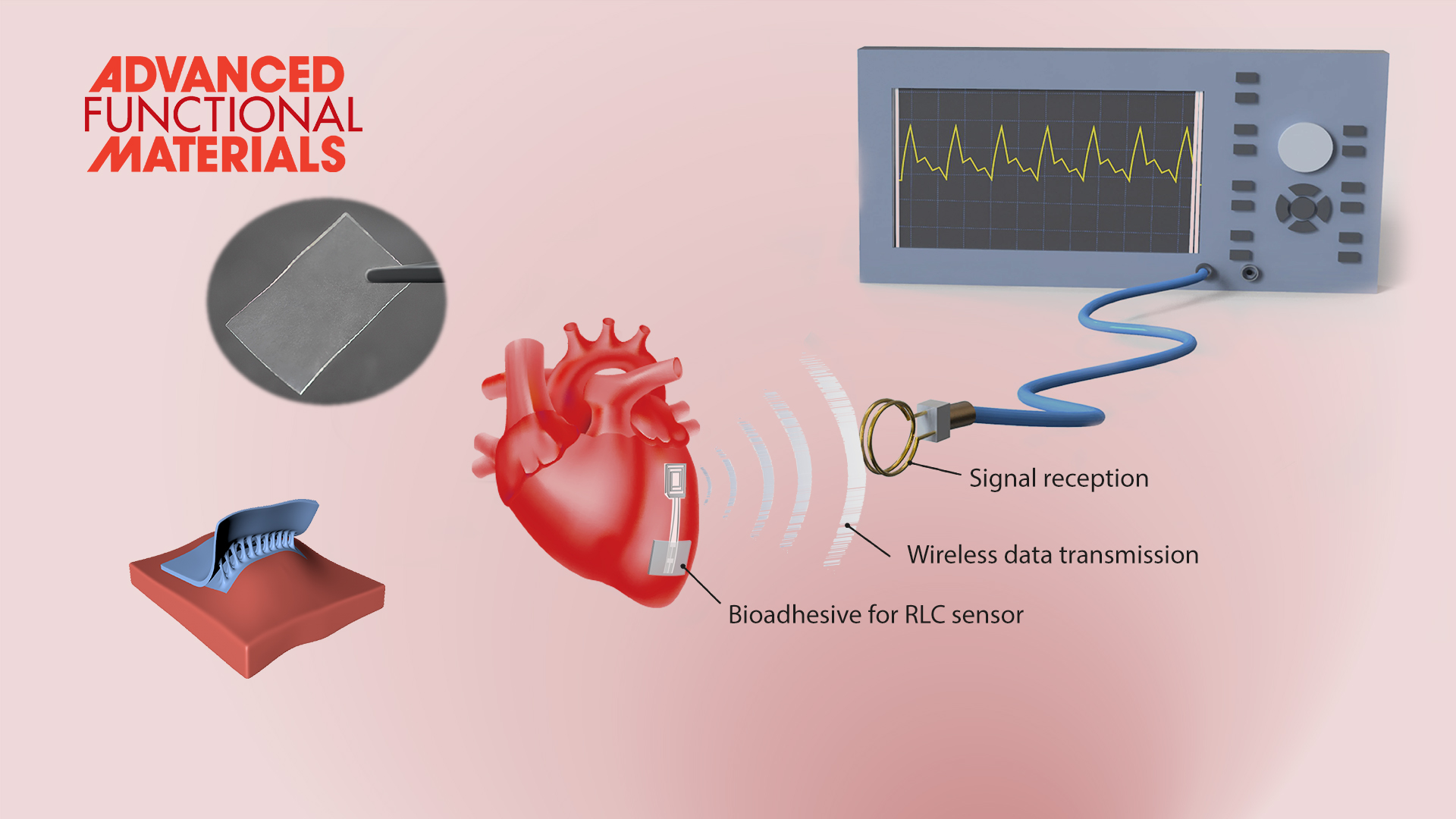
The team further used these hydrogel bioadhesives to build robust biointerfaces for wearable electronic devices, which are capable of forming long-term stable adhesion with various biological tissues to successfully monitor physiological signals such as electromyography, blood pressure, and heart rate. These excellent properties also allow the implanted bioelectronics to be used for real-time physiological and clinical monitoring by fixing the bioelectronics to the animal heart to build a bioelectronic interface between the electronics and the rat heart for wireless detection of biological signals (e.g., blood pressure, heart rate, etc.). Additionally, the hydrogel-based bioelectronics can also be easily detached upon a benign trigger without causing discomfort or damage to the tissues or organs.
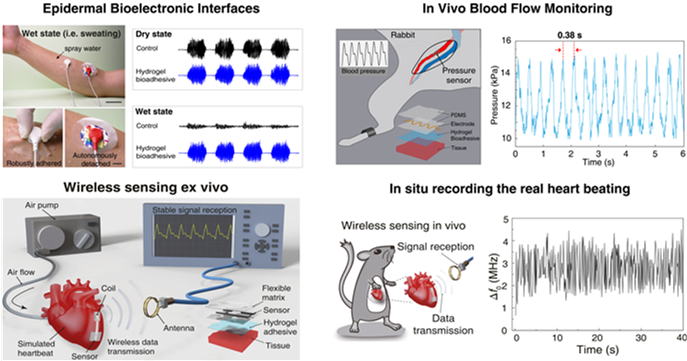
Figure 3. Hydrogel bioadhesives as the ideal material systems to construct the robust interface between electronics and biological tissues.
These bioelectronic adhesive interface developed by Prof. Liu’s team is robust and suitable for wet and dynamic tissue interfaces, providing technical support for the long-term in vivo implantation of bioelectronics.
These studies above were supported by the Shenzhen Municipal Government, SUSTech, and the Centers for Mechanical Engineering Research and Education at MIT and SUSTech (MechERE Centers at MIT and SUSTech). This work was also supported in part by the Science, Technology and Innovation Commission of Shenzhen Municipality, and the Basic and Applied Basic Research Foundation of Guangdong Province.
The authors thank Prof. Fuzeng Ren in the Department of Materials Science and Engineering at SUSTech for his support in cell experiments and Prof. Tao Ye in the Department of Electrical and Electronic Engineering at SUSTech for his support in wireless sensing.
Paper links:
https://onlinelibrary.wiley.com/doi/10.1002/adfm.202111465
https://onlinelibrary.wiley.com/doi/10.1002/adfm.202106446
Ji Liu’s group link:
https://faculty.sustech.edu.cn/?cat=2&tagid=liuj9&orderby=date&iscss=1&snapid=1
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo By