Non-alcoholic fatty liver disease (NAFLD) is a clinicopathological syndrome without a history of excessive drinking, characterized by steatosis and lipid accumulation in liver cells, accompanied by metabolic syndromes such as hyperlipidemia, hyperglycemia, and insulin resistance. It is the most common chronic liver disease. The incidence rate in the world has reached one-third, and it has become a major threat to human health in contemporary society.
Data in 2018 show that the prevalence of NAFLD in China reached 32.9%, of which about 10-20% will further develop into non-alcoholic steatohepatitis, then progress to liver fibrosis, and have a higher risk of developing into severe liver cirrhosis and liver cancer. At present, the pathogenesis of NAFLD is still unclear, and there is no effective clinical drug for the treatment of NAFLD in the world.
Recently, Professor Guozhi Xiao and Associate Professor Huiling Cao’s team from the School of Medicine at the Southern University of Science and Technology (SUSTech) published their findings on utilizing clinical patient samples, transgenic mouse models, and cell and molecular biological methods.
Their research, entitled “Kindlin-2 haploinsufficiency protects against fatty liver by targeting Foxo1 in mice,” was published online in Nature Communications, a multidisciplinary journal covering the natural sciences, including physics, chemistry, earth sciences, medicine, and biology. For the first time, the researchers revealed the important role of Kindlin-2 protein, a key molecule in the adhesion signaling pathway, in the occurrence and development of NAFLD.

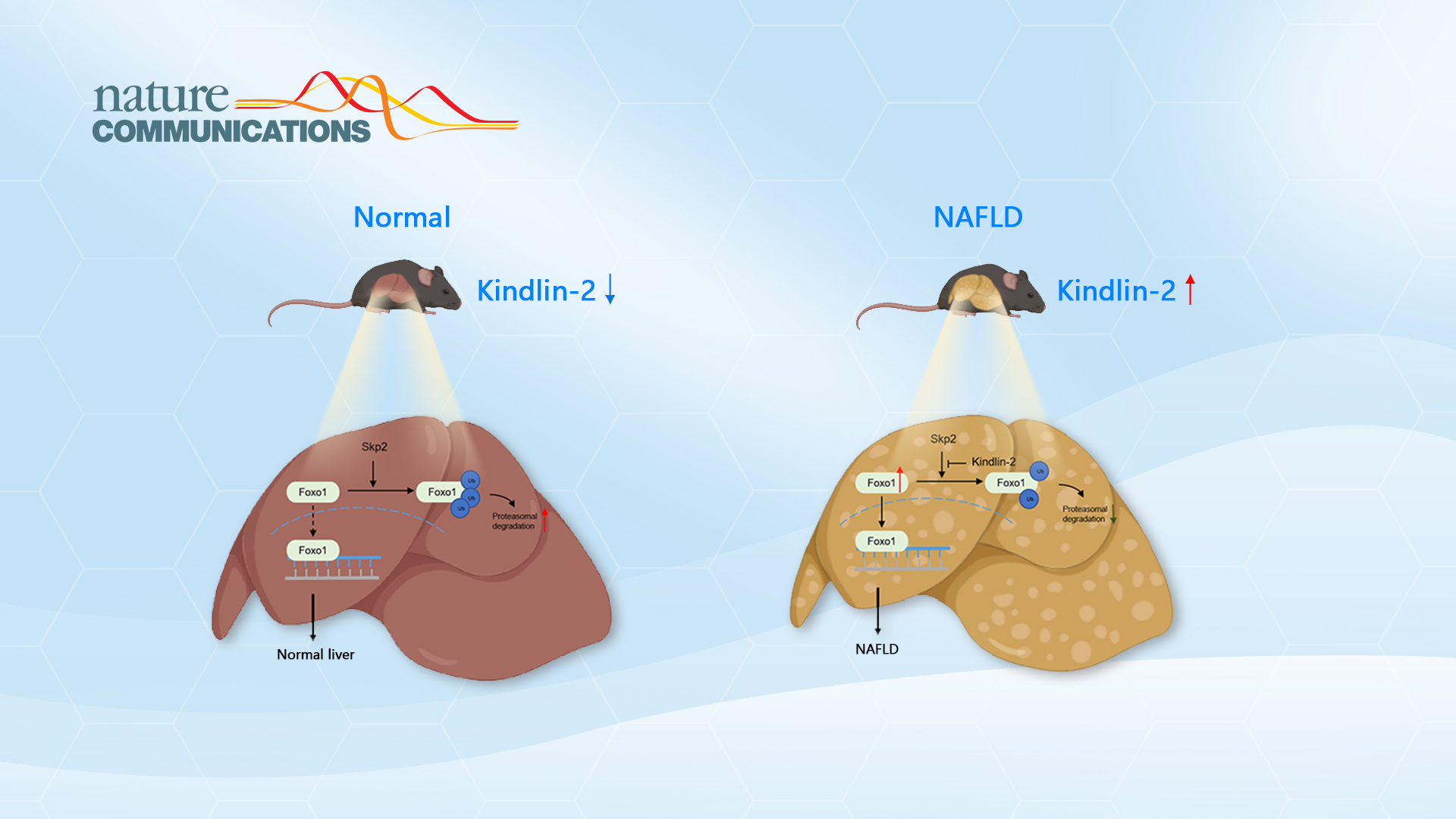
In this study, Prof. Xiao and Cao’s team found that the expression level of Kindlin-2 protein was significantly increased in the liver tissue of NAFLD mice and patients for the first time. In the normal-chow diet, the reduction of Kindlin-2 had no obvious effect on the metabolism of mice, but it could significantly improve NAFLD induced by a high-fat diet. Subsequently, the researchers found that Kindlin-2 could interact with Foxo1 proteins by proteomics.
Molecular biology and biochemical analysis showed that the C-terminal region of Kindlin-2 could bind to Foxo1 and inhibit Skp2-mediated ubiquitination of Foxo1 protein, which enhanced the protein stability of Foxo1. Furthermore, AAV8-mediated knockdown of hepatic Kindlin-2 effectively attenuated NAFLD in mice. This study discovered the important physiological function and regulatory mechanism of Kindlin-2 in liver lipid metabolism for the first time, revealing the important role of Kindlin-2 in the pathogenesis and progression of NAFLD, and provided a new potential therapeutic target for the treatment of NAFLD.
In the past few years, the research group has achieved a series of original scientific research results in the study of organ formation and homeostasis regulation by Kindlin-2. They first demonstrated the important role and new molecular mechanism of Kindlin-2 protein in regulating mesenchymal cell differentiation and skeletal development in 2015 (Wu et al., Nature Communications, 2015).
In 2020, they demonstrated the role and mechanism of Kindlin-2 in promoting pancreatic islet β cell proliferation and regulating β cell insulin secretion (Zhu et al, Nature Communications, 2020) and the vital role of osteocyte Kindlin-2 in regulating bone mass, bone density, and bone remodeling (Cao et al., Bone Research, 2020; Fu et al., Signal Transduction Targeted Therapy, 2020).
The group found that the expression level of Kindlin-2 is significantly decreased in the nucleus pulposus cells of the intervertebral disc in patients with intervertebral disc degeneration (IVDD). Loss of Kindlin-2 in mouse nucleus pulposus cells leads to a severe spontaneous IVDD phenotype and aggravated IVDD injury induced by abnormal mechanical stress, and Kindlin-2 inhibits the activation of the NLRP3 inflammasome pathway in nucleus pulposus cells to maintain the Intervertebral disc homeostasis (Chen et al., Bone Research, 2022). They recently found that Kindlin-2 is highly expressed in articular chondrocytes, and its down-regulation leads to osteoarthritis. The molecular mechanism is related to the up-regulation of Runx2-Stat3 pathway (Wu et al., Nature Aging, 2022).
Dr. Huanqing Gao from the School of Medicine at SUSTech is the first author of this paper. Liang Zhou of Shenzhen University (SZU) and Yiming Zhong and Zhen Ding, both postgraduate students of the School of Medicine at SUSTech, are the co-first authors. Prof. Guozhi Xiao and Associate Prof. Huiling Cao from the School of Medicine at SUSTech are the co-corresponding authors.
This work was supported, in part, by the National Key Research and Development Program of China, the National Natural Science Foundation of China (NSFC), and the Shenzhen Science and Technology Innovation Commission. The authors also acknowledge the assistance of the SUSTech Core Research Facilities.
Paper link: https://www.nature.com/articles/s41467-022-28692-z
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo By