The β-coronavirus family of positive-strand RNA viruses includes severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), SARS-CoV, Middle East Respiratory Syndrome Coronavirus (MERS-CoV), and four seasonal coronaviruses (HCoV-OC43, HCoV-NL63, HCoV-HKU1, and HCoV-229E).
After entering host cells, the genomic RNA of β-coronaviruses is translated into two large polyproteins, which are then cleaved into 16 mature viral proteins, namely nonstructural proteins (nsps) 1-16. Some of the nsps drive the rearrangement of host membranes to form viral replication organelles (ROs) that anchor the viral replication transcription complexes (RTCs). The most prominent ROs induced by β-coronavirus infection are double-membrane vesicles (DMVs). The DMVs provide a favorable microenvironment for protecting newly-synthesized viral products from innate immune surveillance of the host cells. However, the molecular mechanisms, regulation pathways, and host factors involving the DMV formation are still unclear.
Assistant Professor Yan Zhao’s group from the Department of Biology at the Southern University of Science and Technology (SUSTech), in collaboration with Dr. Hongyu Deng’s group at the Institute of Biophysics, Chinese Academy of Sciences (IBP, CAS), reveals that the endoplasmic reticulum (ER)-localized autophagy proteins VMP1 and TMEM41B are critical host factors for β-coronavirus infection. They function at different steps during DMV formation.
Their research results were recently published in the Journal of Cell Biology, entitled “VMP1 and TMEM41B are essential for DMV formation during β-coronavirus infection”.
Both VMP1 and TMEM41B are integral ER-localized proteins essential for autophagosome biogenesis and lipid mobilization. Genetic screens to identify the host proteins required for SARS-CoV-2 infection revealed that TMEM41B and VMP1 are important for virus infection, but the exact mechanism remains unknown. The researchers elucidate that VMP1 and TMEM41B are dispensable for β-coronavirus entry but are required for viral RNA replication. Both proteins play essential roles in DMV formation.
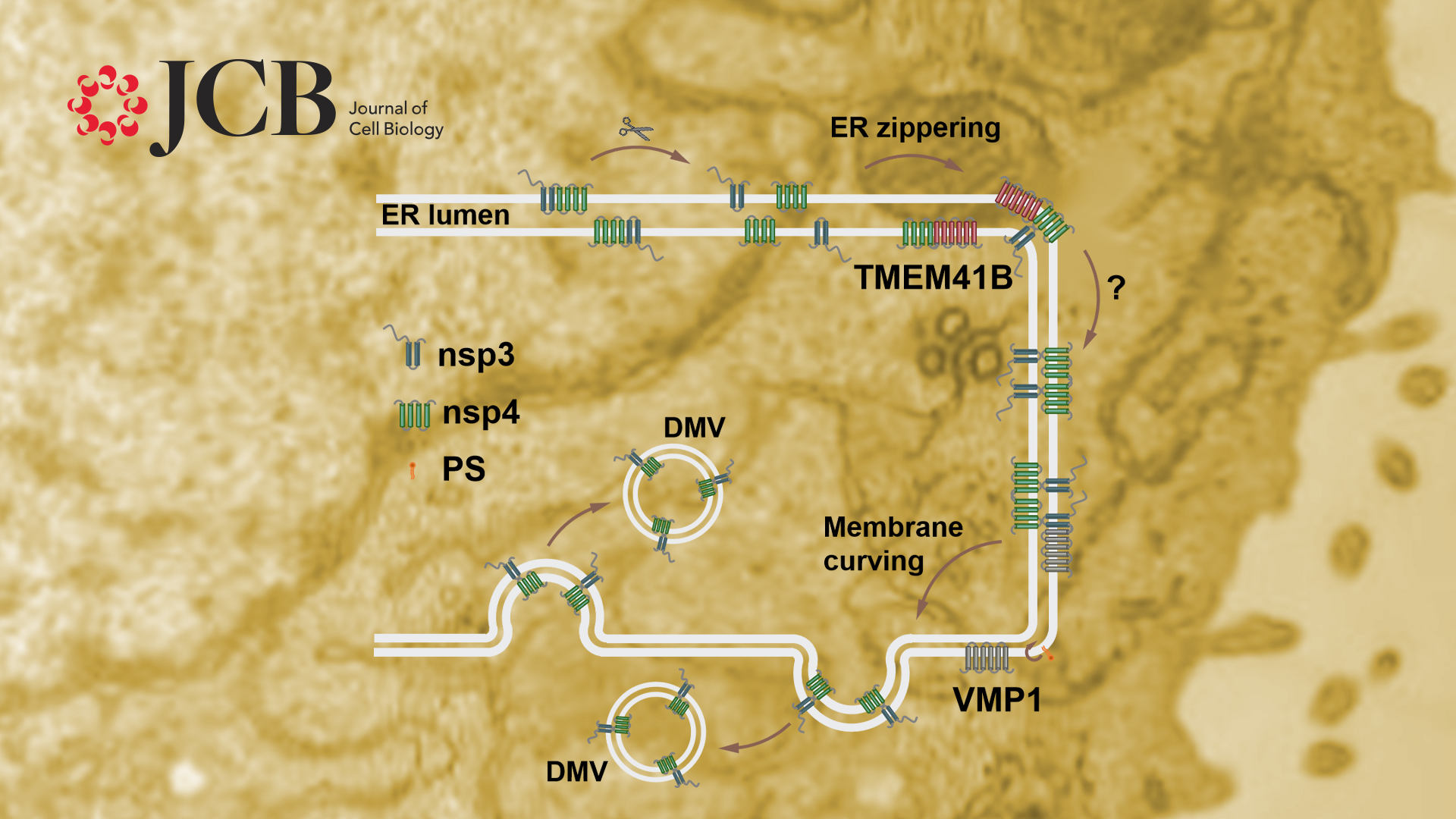
Previous studies revealed that coexpression of β-coronavirus nsp3 and nsp4 induces the formation of DMVs. The researchers verified that ectopic expression of Sars-CoV-2 nsp3 and nsp4 also induce ER zippering through their interaction with their luminal domains, and the paired ER further bends into DMVs. They discovered that nsp3 and nsp4 mainly localize on the outer and inner membranes of DMVs, respectively, and this unique distribution is essential for DMV biogenesis. Using nsp3 and nsp4-induced DMVs as a model, the researchers elucidated that TMEM41B facilitates the interaction between nsp3 and nsp4, and thus promotes the ER paring. VMP1 is dispensable for the zippering of the ER but is essential for generating enough ER membrane curvature to form DMVs. Therefore, VMP1 and TMEM41B play different roles in DMV formation.
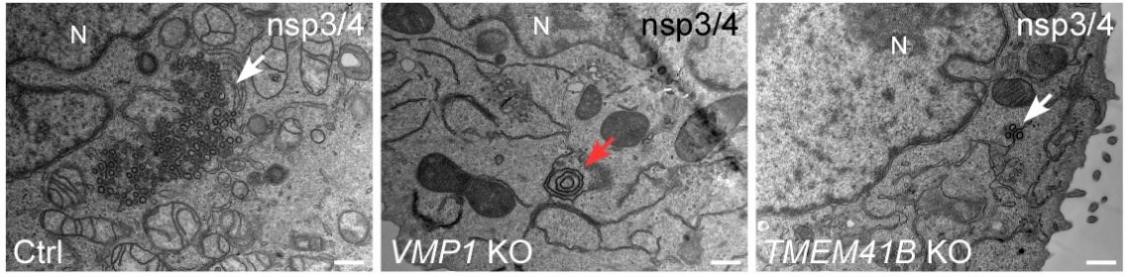
Figure 1. In control (ctrl) cells, ectopic expression of Sars-CoV-2 induces abundant DMV-like vesicles (white arrows). The zippered ER forms concentric structures (red arrow) in VMP1 KO cells, while DMV generation is greatly repressed in TMEM41B KO cells.
VMP1 was shown to regulate cross-membrane phosphatidylserine (PS) distribution. Inhibiting PS synthesis by siPTDSS1 partially rescues the DMV defects in VMP1 KO cells, suggesting that PS participates in DMV formation.
This study reveals that the viral nonstructural proteins nsp3 and nsp4 are localized on two adjacent ER membranes at the DMV formation sites to induce vesicle formation. This unique distribution pattern of nsp3/4 may facilitate bending of the zippered membranes for membrane curvature.
The researchers also discovered distinct functions of VMP1 and TMEM41B at different steps of DMV biogenesis, and dysregulation of PS distribution may contribute to the defects in VMP1 KO cells. These results provide novel insights into how the host factors collaborate with viral proteins to remodel the endomembrane system for virus replication.
Mingming Ji, a postdoctoral fellow, Meng Li, and Long Sun, both Ph.D. students, are the co-first authors of this paper. Asst. Prof. Yan Zhao at SUSTech and Dr. Hongyu Deng at IBP, CAS are the corresponding authors. Assoc. Prof. Yiming Li and postdoctoral fellow Lulu Zhou at SUSTech are the co-authors. Dr. Ying Li at Tsinghua University also provided technical support for this study.
This work was supported by the National Key Research and Development Program, the National Natural Science Foundation of China (NSFC), the Chinese Academy of Sciences, and the Shenzhen–Hong Kong Institute of Brain Science–Shenzhen Fundamental Research Institutions.
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo By