β-1,3-glucan is a core component unique to fungal cell walls and plays a critical role in the viability of a wide range of pathogenic fungi. Echinocandins, developed with the goal of disrupting β-1,3-glucan synthesis, have been widely used as first-line antifungal drugs in clinical practice since their introduction in the 19th century. However, the mechanism of fungal cell wall β-1,3-glucan synthesis and the mechanism of action of echinocandin-based antifungal drugs are still unclear. A series of problems, such as the widespread clinical sources of drug resistance, still plague the scientific field and seriously hinder the development of novel antifungal drugs targeting β-1,3-glucan synthesis.
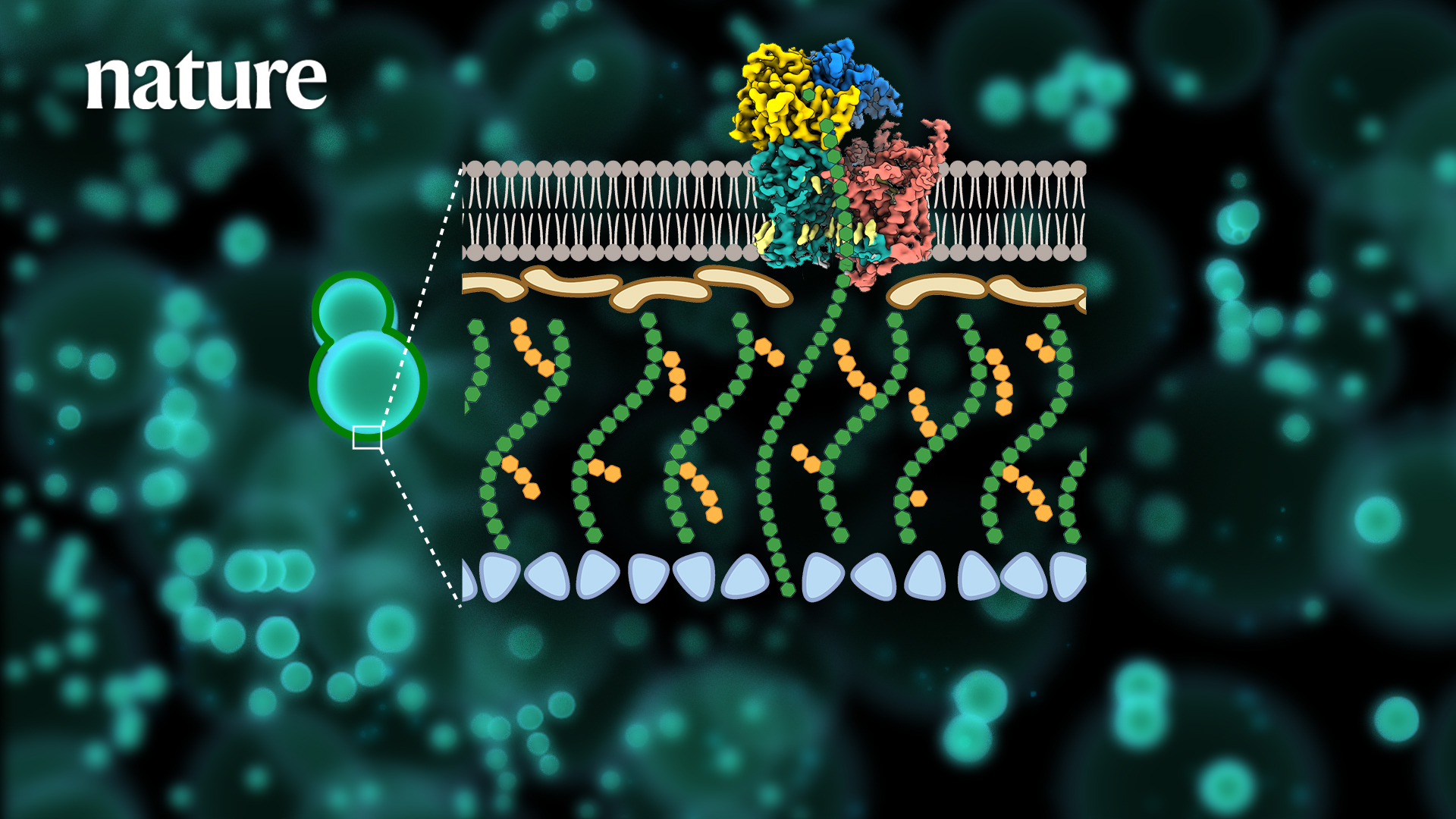
Research Assistant Professor Xiaotian Liu and Chair Professor Mingjie Zhang’s team in the School of Life Sciences at the Southern University of Science and Technology (SUSTech) recently collaborated with Professor Hongjun Yu and Associate Professor Min Zhang from the School of Basic Medical Sciences at Huazhong University of Science and Technology (HUST) to resolve the three-dimensional structure of the key protein machinery for fungal cell wall synthesis and reveal a novel potential mechanism of action of antifungal drugs.
Their research results, entitled “Structural and mechanistic insights into fungal β-1,3-glucan synthase FKS1,” have been published in Nature, one of the world’s leading multidisciplinary science journal.
To address the issues above, the researchers applied single-particle cryo-electron microscopy (Cryo-EM) to resolve the high-resolution structure (3.4 Å) of the fungal β-1,3-glucan synthase FKS1. Its unique molecular panoply is reported for the first time, revealing its key components involved in the β-1,3-glucan synthesis.
Systematic mutagenesis combined with in vivo and in vitro functional analysis further validated the catalytic synthesis and transport mechanism of FKS1. It revealed key amino acid sites conserved in a variety of pathogenic fungi. Next, the researchers resolved the high-resolution electron microscopic structure (3.5Å) of the most common representative clinical drug resistance mutant, FKS1-S643P. Structural comparison analysis revealed that mutations at this site cause significant conformational changes in Y638 and F639 in the resistance mutation hotspot region, and eventually cause rearrangement of nearby lipid molecules, suggesting a unique molecular mechanism for drug resistance generation.
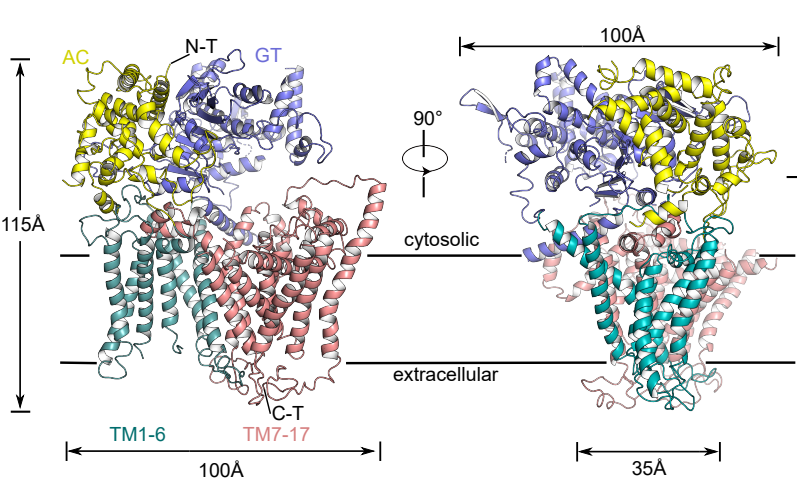
Figure 1. Schematic diagram of the three-dimensional structure of FKS1
More importantly, the research team successfully mapped out the important mutations affecting FKS1 activity as well as the key purification and reaction conditions, and successfully established an efficient and reproducible in vitro reaction system for FKS1 family proteins. For the first time, they eliminated the cumbersome radiolabeling assay, which greatly optimized the drug screening system and filled the gap in the field.

Figure 2. Establishment of an efficient in vitro reaction system and activity assay
In conclusion, this study depicted the three-dimensional structure of fungal glucan synthase FKS1 and successfully elucidated its molecular mechanism. It identifies the key amino acids that undergo drug resistance mutations and their potential resistance mechanisms, which provides a solid foundation for future research and the development of novel antifungal drugs. The reviewers unanimously gave high evaluation to this work and considered it as a major breakthrough in the field.
Profs. Hongjun Yu, Xiaotian Liu, and Min Zhang are the co-corresponding authors of this paper. The research was also assisted by Asst. Prof. Kaige Yan from the School of Life Sciences at SUSTech and Dr. Jing Wu from the Cryo-Electron Microscopy Center at SUSTech.
The research was supported by the Center for Basic Science Program of the National Natural Science Foundation of China (NSFC), Surface Program, and Shenzhen High-Level Talent Team.
Paper link: https://www.nature.com/articles/s41586-023-05856-5
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo By