Phycobilisome (PBS) is the main light-harvesting antennae in cyanobacteria and red algae, located on the stromal side of the thylakoid membrane, which is the largest light-harvesting protein complex. Sunlight is captured by PBS and transferred to the reaction centers of photosystem II (PSII) and photosystem I (PSI) in high efficiency via internal bilins to induce photochemical energy conversion. Although most photosynthetic protein structures have been solved through purification in vitro, the interactions and the energy transfer mechanism in their native state are still unclear.

Professor Sen-Fang Sui’s research group from the School of Life Science at the Southern University of Science and Technology (SUSTech) recently collaborated with Professor Hong-Wei Wang from the School of Life Sciences of Tsinghua University and Professor Xinzheng Zhang from the Institute of Biophysics, Chinese Academy of Sciences (CAS) to jointly reveal the assembly mechanism of the PBS-PSII-PSI-LHC megacomplex in the native state and the efficient energy transfer from PBS to PSII and PSI, which is a milestone in the field of photosynthesis.
Their research work, entitled “In situ structure of the red algal phycobilisome-PSII-PSI-LHC megacomplex,” has been published in Nature.
Prof. Sen-Fang Sui’s research group had previously been working on the structure of PBS. In 2017, they first reported the cryo-EM structure of the complete PBS of the marine red alga Griffithsia pacifica at 3.5 Å resolution, revealing the precise assembly mechanisms of PBS. In 2020, they reported the cryo-EM structure of the complete PBS of Porphyridium purpureum at the 2.8 Å resolution, revealing the energy transfer mechanism within the PBS, which is a breakthrough in photosynthetic research.
Based on previous studies, Porphyridium purpureum was selected for in situ high-resolution analysis. Combining cryo-focused ion beam (cryo-FIB), cryo-tomography (cryo-ET), two conformations of the PBS-PSII-PSI-LHC megacomplex (single-PBS-PSII-PSI-LHC and double-PBS-PSII- PSI-LHC) are solved at the resolutions of 3.3 and 4.3 Å, respectively (Fig. 1). Based on these structures, the researchers discovered some proteins that are not found in the isolated samples, which are crucial for the assembly of the PBS-PSII-PSI-LHC megacomplex. Structural analysis shows that four linker proteins (LRC2, LRC3, LPP1, and LPP2), together with LCM and ApcD, are involved in the binding between PBS-PSII. Thus, red algal PBS could be very stably bound to PSII in vivo.
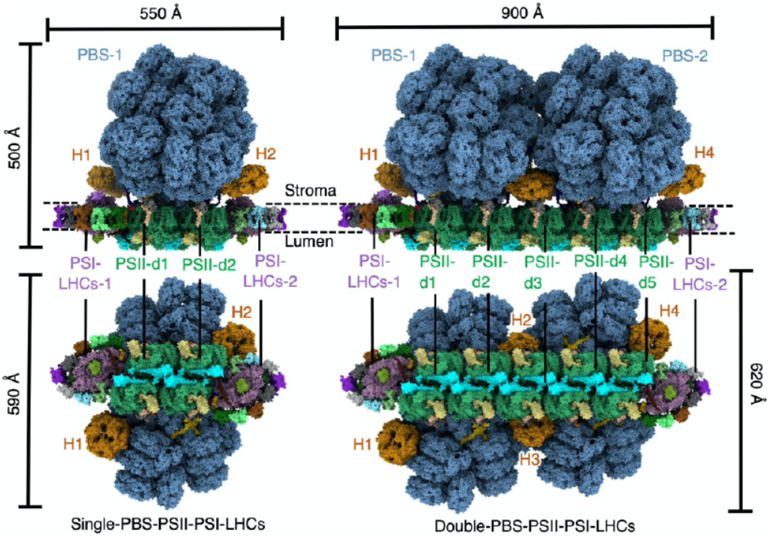
Figure 1. Overall structures of the PBS–PSII–PSI–LHC megacomplex from P. purpureum
Photosynthetic organisms have evolved a mechanism for responding the changing light conditions. The light-harvesting antenna could transfer excess energy to the PSI for energy redistribution to avoid photodamage to the PSII. PBS of cyanobacteria and red algae may transfer energy to PSI through the indirect PBS→PSII→PSI mode and the direct PBS→PSI mode. However, due to the lack of structural information, the energy transfer mechanism between PBS and PSI is still unclear.
The in situ structure of the PBS-PSII-PSI-LHC megacomplex shows that the red algal PBS only interacts directly with PSII. Therefore, the energy would be mainly transferred to PSII, and then transferred to PSI through PSII (Fig. 2). In addition, the researchers also found that there are specially arranged chlorophyll (Chl) clusters in PSII and PSI, which promotes the excited coupling state of the π electrons on the conjugated ring, reducing the energy level to ensure efficient energy transfer (Fig. 2).
In summary, this work proves that red algae transfer energy to PSI through the indirect PBS→PSII→PSI to redistribute the energy between PSII and PSI. This achievement also provides a new theoretical basis for artificially simulating photosynthesis research.
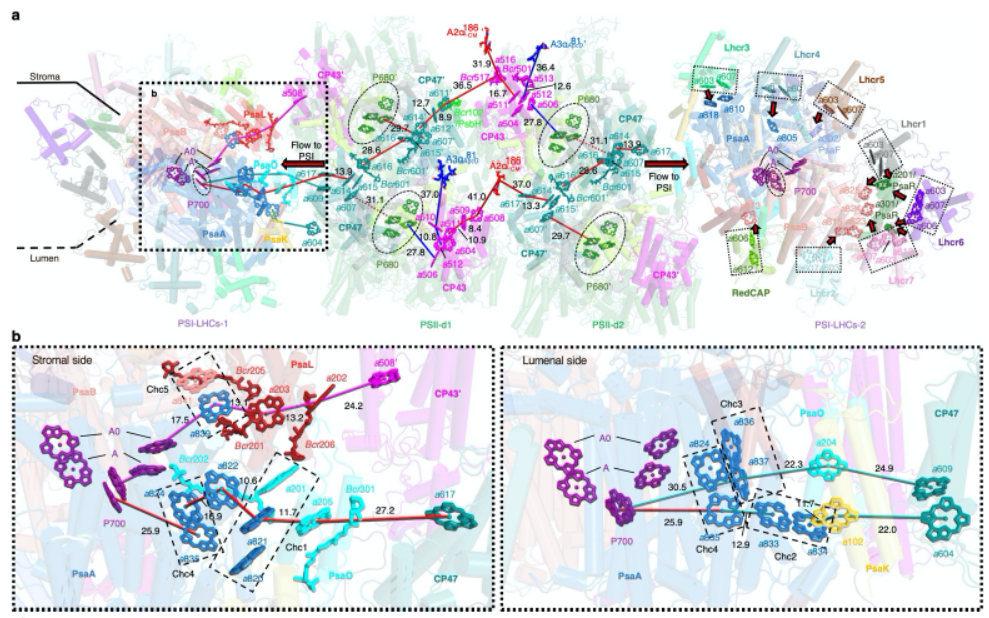
Figure 2. Key pigment arrangements and possible energy transfer pathways
Xin You, a Ph.D. candidate from Tsinghua University, Xing Zhang, a postdoctoral fellow from Tsinghua University, Jing Cheng, a postdoctoral fellow from the Institute of Biophysics, CAS, and Yanan Xiao, a postdoctoral fellow from SUSTech, are the co-first authors of this paper. Prof. Sen-Fang Sui, Prof. Hong-Wei Wang, and Prof. Xinzheng Zhang are the co-corresponding authors.
Dr. Jianfei Ma from the School of Life Sciences at Tsinghua University contributed to the preliminary exploration. Prof. Shan Sun from Tsinghua University and a visiting scholar at SUSTech also participated in the structural analysis.
Paper link: https://www.nature.com/articles/s41586-023-05831-0
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo By