Consciousness is the most important and sophisticated function of the human brain. How is consciousness generated? How does consciousness disappear? How does consciousness emerge after disappearing? We still lack a deep understanding of its biological mechanisms. Among the 125 major scientific issues listed in the journal Science in 2016, the first one is the composition of the universe, and the second is the biological basis of consciousness.
Professor Xue-Jun Song’s research group from the School of Medicine at the Southern University of Science and Technology (SUSTech) has recently published their research findings on elucidating the molecular and neural mechanisms for the reboot of consciousness from anesthesia. It unravels the tip of the iceberg of the biological basis of consciousness, consciousness disturbance, and consciousness recovery.
Their research work, entitled “Emergence of consciousness from anesthesia through ubiquitin degradation of KCC2 in the ventral posteromedial nucleus of the thalamus,” has been published in Nature Neuroscience.
Since the first application of general anesthetics in 1840, the reversible loss of consciousness induced by anesthetics has been used as the best biomedical model for studying the loss of consciousness and its recovery. For a long time, the widely accepted view on the recovery of consciousness is that it is a passive process completely dependent on the reduction of anesthetics in the brain; that is, when the anesthetics are reduced to a certain level in the brain tissue, consciousness will naturally recover.
In recent years, the view of the purely passive recovery of consciousness has been challenged. Scientists began to explore the neural circuit mechanisms of consciousness recovery and found that activating certain neural circuits can promote the recovery of consciousness. It suggests that these circuits are related to consciousness recovery and may play a role in promoting consciousness reactivation. However, the research to date has not touched on the essence of consciousness recovery.
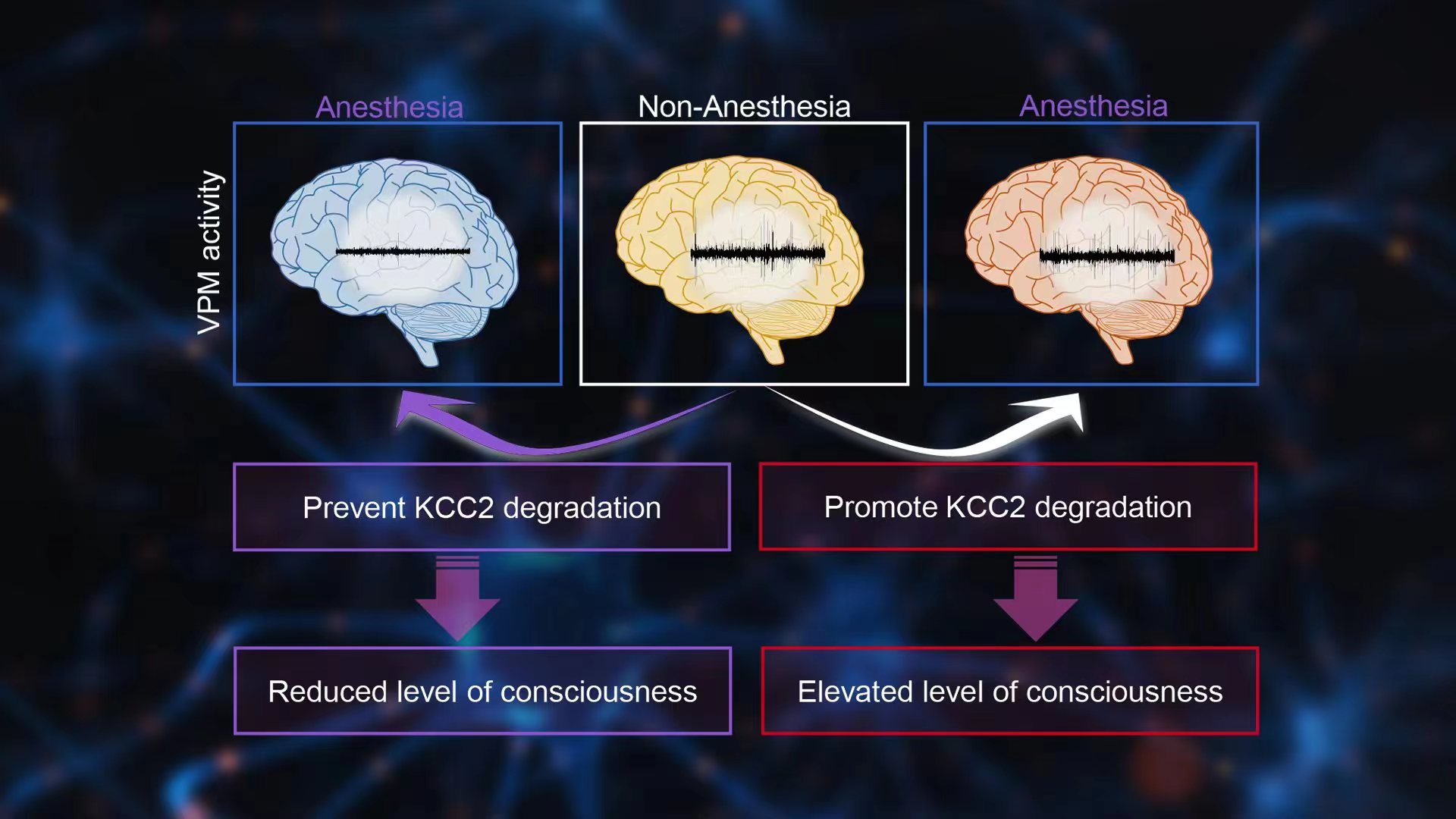
The research from Prof. Xue-Jun Song’s lab at SUSTech pointed out that the recovery of consciousness is an active process, not a passive process. The so-called passive recovery is an easily observable, intuitive, and superficial phenomenon, but not the essence of consciousness recovery. The active process of consciousness recovery is based on the intrinsic power of the brain and initiated by the ubiquitin degradation of KCC2 that is controlled by phosphorylation of its Thr1007 site in the ventral posteromedial nucleus (VPM) of the thalamus. The degradation of KCC2 in VPM neurons leads to the disinhibition of the GABAA receptor, thereby accelerating the recovery of VPM neuron excitability and awakening from unconsciousness.
The ubiquitin degradation of KCC2 in VPM is a common molecular mechanism for active recovery of consciousness, which is independent of the pharmacological properties and molecular targets of general anesthetics, including the NMDA receptors and GABAA receptors. Disinhibition of VPM neurons induced by KCC2 degradation may trigger and initiate the formation of highly sensitive local neural networks that renders high-quality communications between these local neural networks and initiates the reconstruction of global neural networks.
The Nature Neuroscience editor’s summary marks that “this study reveals a common mechanism for active reboot of consciousness from anesthesia”. This research has received high appreciation and very positive responses from worldwide neuroscientists and some scientific public media.
Jiangjian Hu, a Ph.D. candidate at SUSTech, is the first author of this paper. Yuexin Liu and Hongyu Yao, both of whom are Ph.D. candidates at SUSTech, are the co-first authors, and Prof. Xue-Jun Song is the corresponding author.
The research was supported by the National Natural Science Foundation of China (NSFC), Shenzhen Science and Technology Program, Shenzhen Science and Technology Innovation Commission, and SUSTech.
Paper link: https://doi.org/10.1038/s41593-023-01290-y
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo By