Boron dipyrroles (BODIPYs) are N, N π – conjugated tetracoordinated organic boron compounds that are chelated by bipyrroles. They have advantages such as high fluorescence quantum yield, high sensitivity, high molar extinction coefficient, high photostability, and insensitivity to acids and bases. They are widely used in fields such as fluorescence probes, biological imaging, photoelectric materials, and photocatalysts.
Considering their great importance and enormous potential applications, and their significance in chiral chemistry in the fields of biomedicine and functional materials, chemists have also attempted to introduce chiral elements, such as carbon center chirality, axial chirality, and helical chirality into the skeleton of BODIPYs. These novel chiral BODIPYs fluorescent molecules have shown good application prospects in the fields of chiral functional materials and chiral catalysts. So far, only two cases of boron-stereogenic BODIPYs have been reported via the resolution of racemates using chiral high-performance liquid chromatography. Yet, examples of obtaining boron chiral BODIPY through asymmetric catalysis have not been reported.
Associate Professor Chuan He’s research team from the Department of Chemistry at the Southern University of Science and Technology (SUSTech) has recently published a breakthrough in boron-stereogenic BODIPY by reporting a palladium-catalysed protocol for the enantioselective synthesis of boron-stereogenic BODIPYs via a desymmetric intramolecular C–H arylation reaction.
Their research work, entitled “Catalytic Enantioselective Synthesis of Boron-Stereogenic BODIPYs,” has been published in Nature Synthesis.
Prof. He’s group has been conducting systematic research on the synthesis and application of heteroatoms chiral compounds (silicon, boron, sulfur, etc.). Based on previous research, the team envisioned using the delocalization characteristics of BODIPYs, combined with theoretical calculations, to achieve a series of novel boron-centered chiral N2O-BODIPYs with high efficiency and enantioselectivity through intramolecular hydrocarbon arylation reactions. This asymmetric catalytic reaction can not only obtain boron chiral N2O-BODIPYs with six-membered rings, but also obtain target products with good enantioselectivity for seven, eight, and nine-membered rings (Figure 1).
DFT calculations have shown that the oxidative addition process is a key step in inducing chiral production. At the same time, by comparing the energy of key transition states, it is shown that the enantioselectivity of the reaction is the result of the co-action of steric hindrance and attractive interaction between ligand and substrate.
This research work achieved the first asymmetric catalytic synthesis of boron chiral BODIPYs through a theoretically guided and experimentally confirmed mode. These novel boron chiral BODIPYs exhibit excellent optical properties and good application prospects in the field of chiral recognition. This method provides a new approach for the synthesis of boron chiral BODIPYs and opens up a new development direction for the study of chiral boron chemistry.
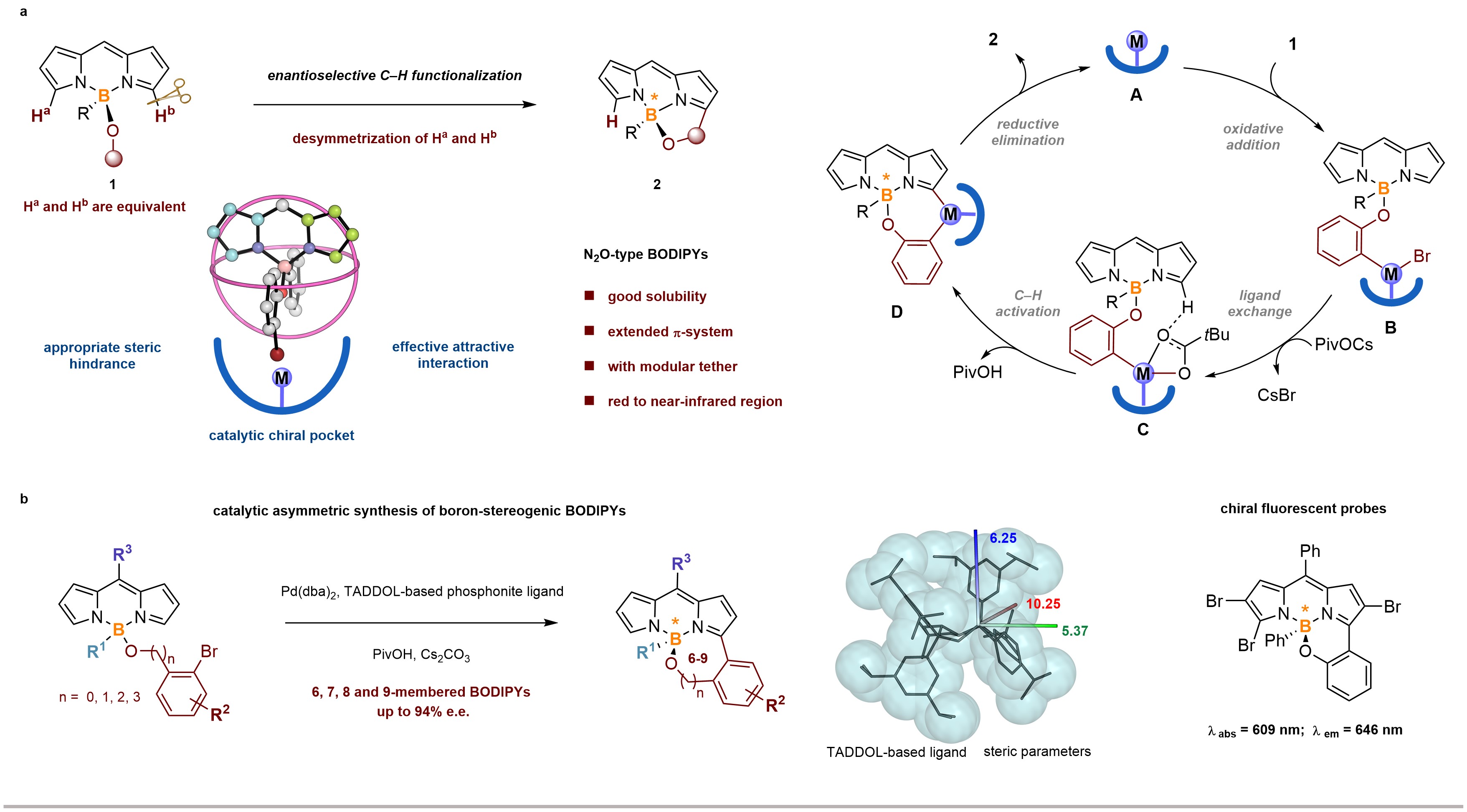
Figure 1. Catalytic Enantioselective Synthesis of Boron-Stereogenic BODIPYs
Bing Zu and Yonghong Guo, doctoral candidates from SUSTech, are the co-first authors of this paper. The co-corresponding authors include Dr. Yingzi Li of Colorado State University and Assoc. Prof. Chuan He of SUSTech.
This research work was supported by the National Natural Science Foundation of China (NSFC), Shenzhen Science and Technology Innovation Commission, Shenzhen Nobel Prize Scientists Laboratory Project, Guangdong Provincial Key Laboratory of Catalysis, and the SUSTech Core Research Facilities .
Paper link: https://www.nature.com/articles/s44160-023-00262-1.
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo By