Alkenes are extremely important feedstocks that serve as precursors and intermediates for the synthesis of value-added targets. Transition metal-catalyzed hydrofunctionalizations of alkenes represent one of the most atom- and step-economic strategies to construct C-C/C-X (X = N, O, B, Si…) bonds. However, hydrofunctionalizations of unactivated alkenes remain a formidable challenge due to the low reactivity, competitive side reactions, as well as difficulties associated with regio-, chemo-, and stereoselectivity control.
Associate Professor Wei Shu’s research group from the Department of Chemistry at the Southern University of Science and Technology (SUSTech) has recently achieved a series of progress in first-row transition-metal-catalyzed regio- and enantioselective hydrofunctionalizations of unactivated alkenes. The relevant results have been published in some of the top academic journals, such as Angewandte Chemie, Chem Catalysis, and ACS Catalysis.
Aliphatic amines are prevalent in pharmaceutical molecules, organic materials, and high-value fine chemicals. Accordingly, how to efficiently synthesize alkyl amines is one of the most important research directions in organic synthesis. The regional divergent synthesis strategy can obtain multiple types of products by changing the minimum reaction parameters, and is one of the most efficient and green synthesis strategies. The research group developed a catalyst-regulated regiodivergent hydroalkylation of unactivated olefins (Scheme 1). Starting from identical olefins, α -/β- branched-, and linear hydroalkylations of olefins have been achieved using different first-row transition-metal and ligands.
This research was published in Angewandte Chemie under the title of “Orthogonal Access to α-/β-Branched/Linear Aliphatic Amines by Catalyst-Tuned Regiodivergent Hydroalkylations”.
Peng-Fei Yang, a Ph.D. student from the Department of Chemistry at SUSTech, is the first author of this paper. Prof. Wei Shu is the corresponding author, while SUSTech is the sole corresponding unit.

Figure 1. Catalyst-tuned regiodivergent hydroalkylations
Chiral C(sp3)-C(sp3) bonds are important substructures that play an essential role in pharmaceutical biomolecules, natural products, and functional material molecules. How to efficiently build the chiral C(sp3)-C(sp3) keys remains a challenge. Prof. Shu’s group developed the Ni-asymmetric hydroalkylation of non-activated olefins with alkyl halides, providing a general and practical access to fully alkyl substituted tertiary stereogenic carbon centers remote to activating groups (Scheme 2). This reaction undergoes the regio- and stereoselective hydrometalation of unactivated alkenes with a nontrivial Markovnikov selectivity, followed by the cross-coupling with unactivated alkyl electrophiles to access trialkyl tertiary saturated stereogenic centers remote to activating groups.
This research was published in ACS Catalysis under the title of “Regio- and Enantioselective Hydrocarbofunctionalizations of Unactivated Olefins Enabled by Nickel Catalysis: Reaction Development and Mechanistic Insights”.
Peng-Fei Yang is the first author of this paper. Dr. Lei Zhu, a postdoctoral fellow at the Army Medical University, is the co-first author of the paper. Prof. Qin Ouyang of the Army Medical University and Prof. Wei Shu are the corresponding authors, while SUSTech is the first affiliation unit.
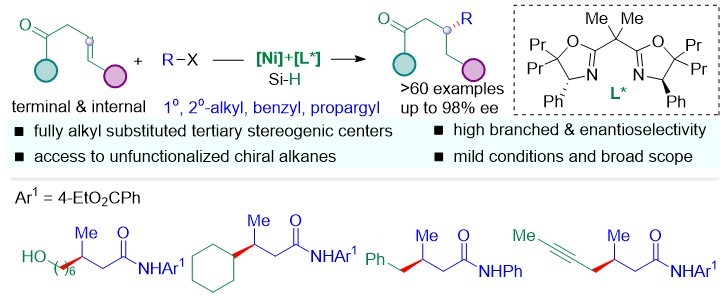
Figure 2. Regio- and enantioselective hydroalkylations of unactivated olefins
Enantioenriched 1,2- and 1,3-diamines with chiral α-branched aliphatic amine motifs is important substructures in bioactive compounds and related molecules and serve as privileged chiral ligands in both organo- and transition-metal-catalysis. However, direct access to such structural motifs remains challenging. The research group developed a straightforward method to access 1,n-diamines (n = 2, 3, 4) containing chiral α-branched aliphatic amine by Ni-catalyzed asymmetric hydroamination of unactivated aliphatic alkenes (Scheme 3). Facilitated by a remote weakly coordinating group, the reaction is applicable to both terminal and internal unactivated alkenes, delivering enantioenriched 1,2-, 1,3-, and 1,4-diamine precursors in good yields and excellent enantioselectivities with diverse substitution patterns.
This research was published in ACS Catalysis under the title of “Access to Enantioenriched 1, n-Diamines via Ni-Catalyzed Hydroamination of Unactivated Alkenes with Weakly Coordinating Groups”.
Recently, an invited perspective in Ni-catalyzed hydrofunctionalizations of alkenes was published in Chem Catalysis under the title of “Asymmetric Alkyl-Alkyl Cross-Coupling Enabled by Earth-Abundant Metal-Catalyzed Hydroalkylations of Olefins”.
Peng-Fei Yang is the first author of this paper. Prof. Wei Shu is the corresponding author, while SUSTech is the sole corresponding unit.
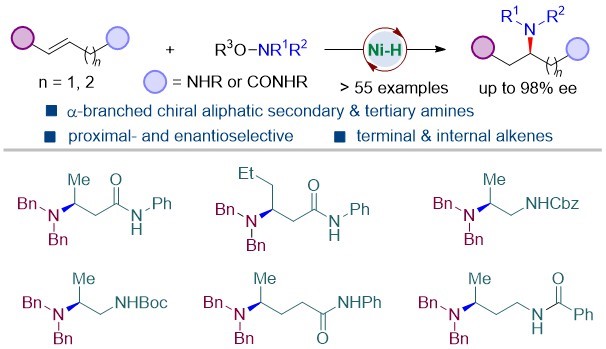
Figure 3. Regio- and enantioselective hydroamination of unactivated olefins
This research was supported by the National Natural Science Foundation of China (NSFC), Guangdong Basic and Applied Basic Research Foundation, Science, Technology and Innovation Commission of Shenzhen Municipality, and Guangdong Provincial Key Laboratory of Catalysis.
Paper links (In order of appearance above):
Angewandte Chemie: https://doi.org/10.1002/anie.202208018
ACS Catalysis: https://doi.org/10.1021/acscatal.2c00665
ACS Catalysis: https://doi.org/10.1021/acscatal.2c02892
Chem Catalysis: https://doi.org/10.1016/j.checat.2023.100508
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo By