Nuclear pore complex (NPC), one of the largest proteinaceous machines in eukaryotes, mediates the nucleocytoplasmic transport of macromolecules and participates in many important cellular processes. In recent years, the intricate 3D architectures and fine structures of the NPC are now being unveiled by combing cryo-EM technology, x-ray crystallography, mass spectrometry, and artificial intelligence. However, the molecular mechanism of its nucleocytoplasmic transport remains largely unknown.
As one of the most representative macromolecular assemblies transported through NPC, ribosome biogenesis is an energy-consuming and orderly regulated process involving more than 200 ribosome assembly factors (AFs). The assembly of ribosomal subunits starts in the nucleolus, followed by a series of sequential processes in both nucleolus and nucleoplasm, and the final maturation takes place in the cytoplasm.
Numerous structures of the pre-60S ribosomal particles have been reported, nearly covering all the major steps of the nuclear and cytoplasmic assembly stages. However, these structures only contain very limited structural information for the export process, which greatly hinders our understanding of the export of the pre-60S particles through the NPC at a molecular level.
Not long ago, Professor Sen-Fang Sui’s research team from the School of Life Sciences and Cryo-Electron Microscopy Center at the Southern University of Science and Technology (SUSTech) determined the structure of yeast NPC by cryoelectron microscopy single particle technology and first solved the near-atomic structure of inner ring (IR) at 3.73 Å, which is the most detailed and accurate model of IR thus far (Cell Research 2022). Based on this previous research, this team has recently determined the high-resolution structure of pre-60S trapped in the central transport channel of NPC, observed the conformational changes of pre-60S upon nuclear export, and unveiled the translocation mechanism of pre-60S unclear export through NPC.
This research work, entitled “Nuclear export of the pre-60S particles through nuclear pore complex”, has been published in Nature.
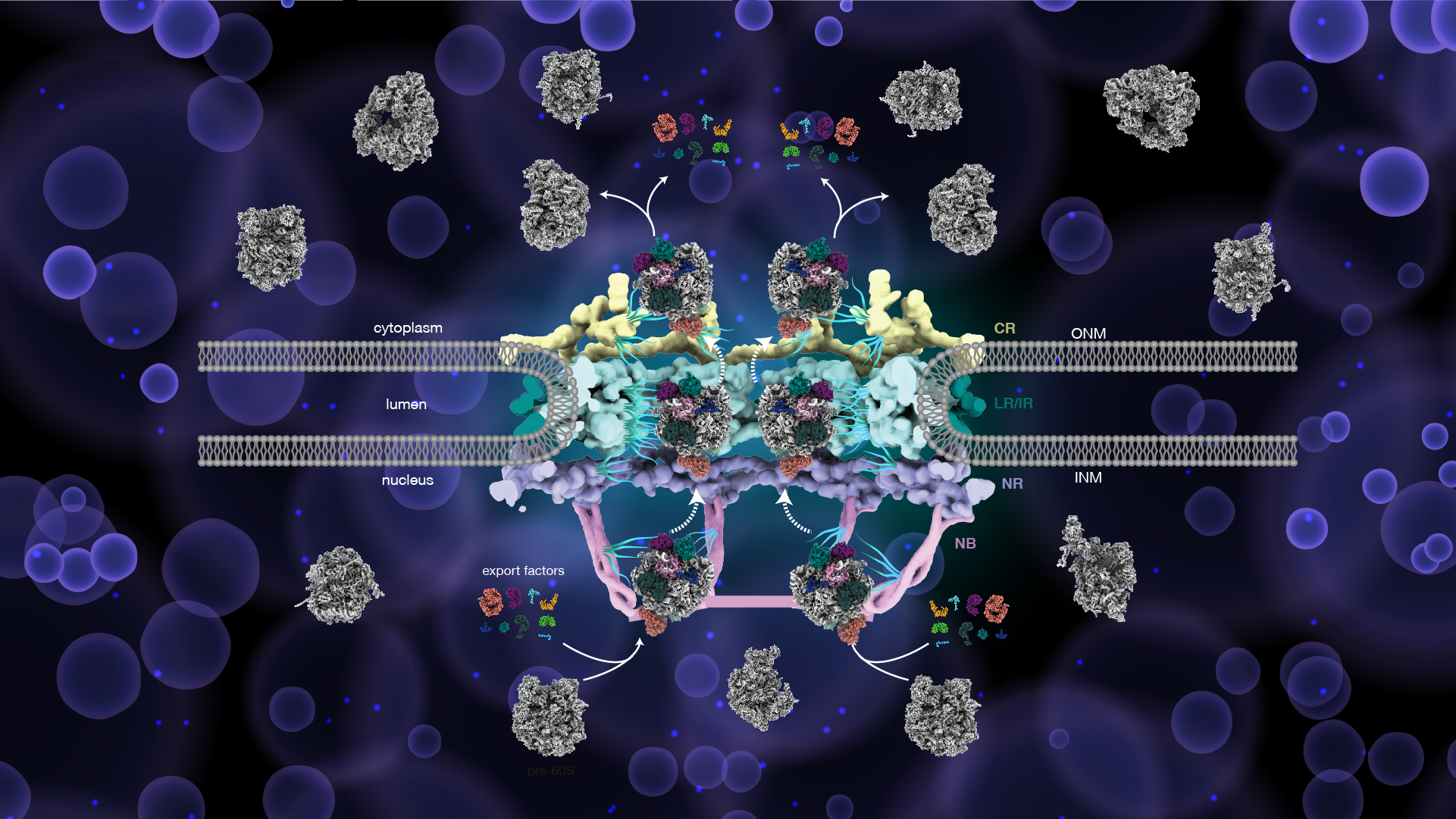
In this study, by combining biochemistry and cryoelectron microscopy, the high-resolution structure of pre-60S in the process of nucleocytoplasmic transport was successfully resolved at 2.64 Å (Fig. 1a-c). Almost all nuclear exporters reported so far were bound to the surface of the pre-60S particle, demonstrating the nuclear export of pre-60S is a highly energy-consuming and complicated process (Fig. 1c-d).

Figure 1. The native structure of pre-60S particle trapped in the NPC channel
Structural analysis suggested that pre-60S shows obvious differences before and after nuclear export as follows: Compositionally, it shows the binding and disassociation of numerous assembly factors and nuclear exporters. Structurally, it also exhibits dramatic conformational changes in the L1 stalk, H38, ES27, and Peptidyl Transferase Centre (PTC) regions (Fig. 2).
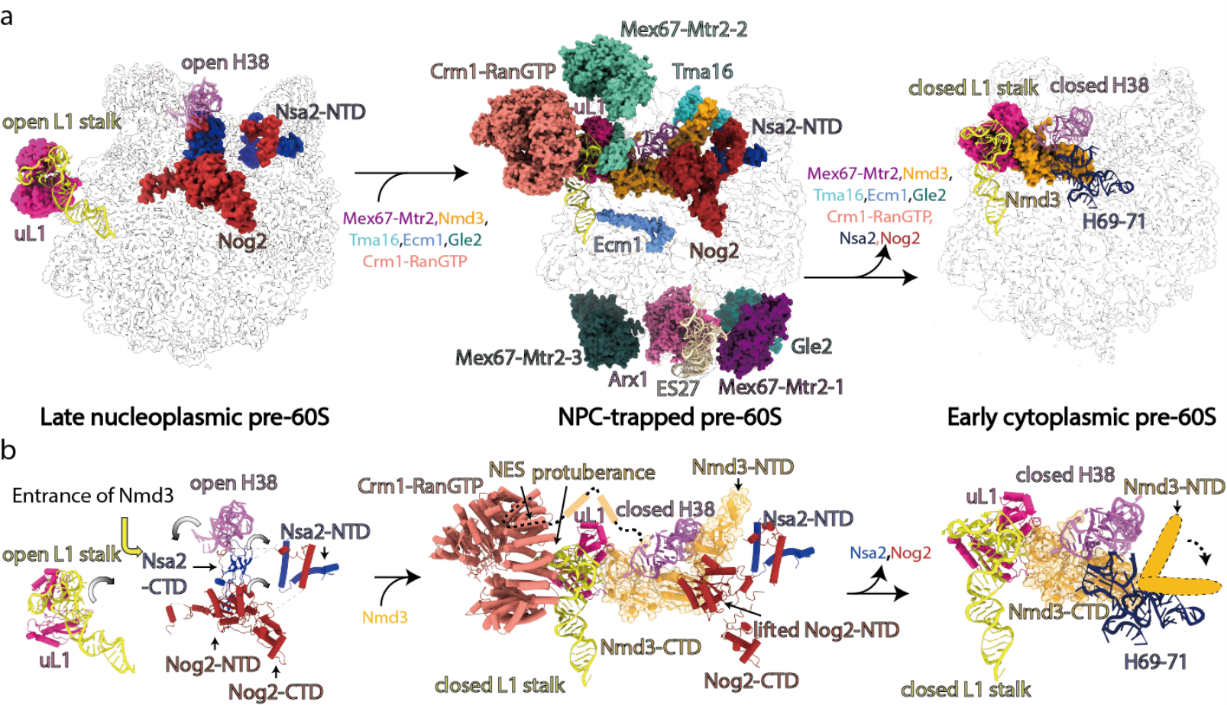
Figure 2. Conformational changes of pre-60S particle upon nuclear export
In addition, the aforementioned nuclear exporters are mainly located on the subunit interface of pre-60S, which mediates the interaction between pre-60S and NPC through the association with the FG-repeats omitted from the NPC scaffold and promote the nuclear export of pre-60S by shielding the negative charge on the rRNA surface (Fig. 3a-c). The distribution of pre-60S and its surface-bound exporters in the NPC indicates that the subunit interface of pre-60S is oriented and adheres to the wall of nuclear pores and that IR may be a key quality checkpoint upon unclear export (Fig. 3d-g).
Another interesting finding is that the NPC could translocate more than one pre-60S particle simultaneously, and up to four pre-60S particles were also observed to reside in the same NPC, which is necessary to meet the physiological needs of the highly active cells (Fig. 3h). Based on the above observations, the researchers proposed a plausible model of the pre-60S translocation through the NPC (Fig. 3i).
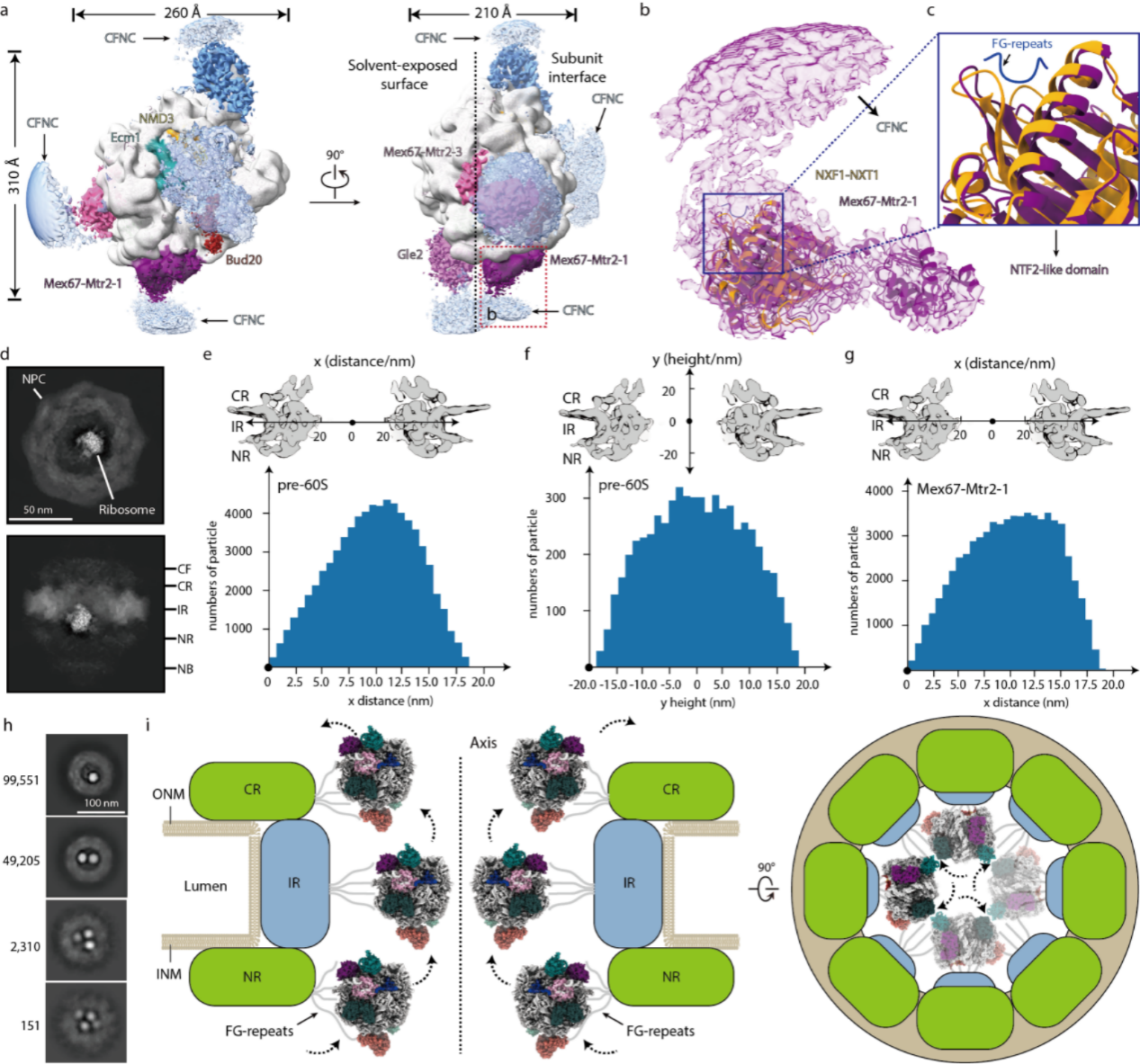
Figure 3. Model of the pre-60S particle translocation through the NPC
These results provide a solid structural basis for further improving the ribosome biogenesis and further understanding the powerful function of NPC, and have a significant role in promoting the cross-research of ribosome and NPC. At the same time, it is also of great significance to understand the pathogenesis of related diseases and to develop corresponding treatment regimens and specific drugs.
Zongqiang Li, a postdoctoral fellow from Tsinghua University and visiting scholar at SUSTech, and Shuaijiabin Chen, a Ph.D. student from Tsinghua University and visiting student at SUSTech, are the co-first author of this paper. Prof. Sen-Fang Sui is the corresponding author.
Liang Zhao, a postdoctoral fellow from Tsinghua University and visiting scholar at SUSTech, Guoqiang Huang, a Ph.D. student from Tsinghua University, Huiqin Xu, a Ph.D. student from SUSTech, Xiaoyun Yang, Research Assistant Professor from SUSTech, Peiyi Wang, Professor of the Cryo-Electron Microscopy Center at SUSTech, and Ning Gao, Professor of Peking University (PKU), also participated in this study.
Shan Sun, Professor of Tsinghua University and visiting scholar at SUSTech, Yaowang Li, Research Assistant Professor from SUSTech, and Yunyang Zhang, a Ph.D. student from PKU, provided valuable suggestions for this work.
This research was supported by the National Natural Science Foundation of China (NSFC) and the Tsinghua-Peking Center for Life Sciences. The data collection and image processing of this study were supported by the Cryo-Electron Microscopy Center of SUSTech.
Paper link: https://rdcu.be/ddrP6
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo By