Natural products and their derivatives have long been the main source of innovative drugs and an important tool for biomedical research. Nearly 60% of clinically applied drugs are directly or indirectly derived from natural products. However, the source of natural products is very limited, and it is difficult to meet the needs of various research. Therefore, the research on the synthesis of active natural products is crucial for the in-depth development of Chinese medicine modernization.
Professor Chuang-Chuang Li’s research group from the Department of Chemistry at the Southern University of Science and Technology (SUSTech) has recently published multiple works that used [5+2] cycloaddition as the vital step to complete the first total syntheses of Daphnillonin B, Daphgraciline, and Bufogargarizins. His group has been committed to the total synthesis of active natural products and has developed a unique [5+2] reaction.
They have published three papers in the Journal of the American Chemical Society (JACS), a high-impact academic journal covering all of chemistry and interfacing areas of science.

Professor Chuang-Chuang Li (first from the right) and his team
[5+2] New breakthrough: the first total synthesis of alkaloid DaNB
Daphnillonin B, which was first isolated from Daphniphyllum longeracemosa by Yue and co-workers in 2019, is an example of such a daphnicyclidin-type alkaloid. Structurally, daphnillonin B has a new [7-6-5-7-5-5] A/B/C/D/E/F hexacyclic core, with a unique bridged azabicyclo[4.3.1] A/B ring system. Additionally, daphnillonin B contains one sterically hindered tetrasubstituted double bond (C8=C13) and eight stereocenters, including one all-carbon quaternary stereocenter at C5 and one tertiary alcohol at C10. Therefore, the total synthesis of Daphnillonin B poses a daunting challenge.
Prof. Li’s group has achieved the first total synthesis of (±)- and (−)-daphnillonin B, a daphnicyclidin-type alkaloid with a new [7-6-5-7-5-5] A/B/C/D/E/F hexacyclic core, with a longest linear sequence of 28 steps, from commercially available staring material (ocresol). A mild type I intramolecular [5+2] cycloaddition enabled the diastereoselective and efficient synthesis of the [5-7] C/D ring system. A Grubbs II catalyst-catalyzed radical cyclization allowed diastereoselective construction of a bridged B ring. A diastereoselective intramolecular Pauson−Khand reaction efficiently installed the desired [5-5] fused E/F ring system. Notably, the unique Wagner−Meerwein-type rearrangement enabled the efficient construction of the synthetically challenging [7-6-5-7-5-5] hexacyclic core from the [6-6-5-7-5-5] hexacyclic framework, which is found in calyciphylline A-type Daphniphyllum alkaloids.
This approach could be applied to synthesize other members of the calyciphylline A-type and daphnicyclidin-type subfamilies of alkaloids and their analogs, enabling further biological research.

Figure 1. The first total synthesis of Daphnillonin B. Image credit: JACS
Senior Research Scholar Yunpeng Zou and postgraduate fellow Zhenglin Lai are the co-first authors of this paper. Professor Chuang-Chuang Li is the sole corresponding author, and SUSTech is the only affiliated unit. Research Assistants Mengwei Zhang and Shuai Ning, and doctoral student Jianzhao Peng also made important contributions to the work.
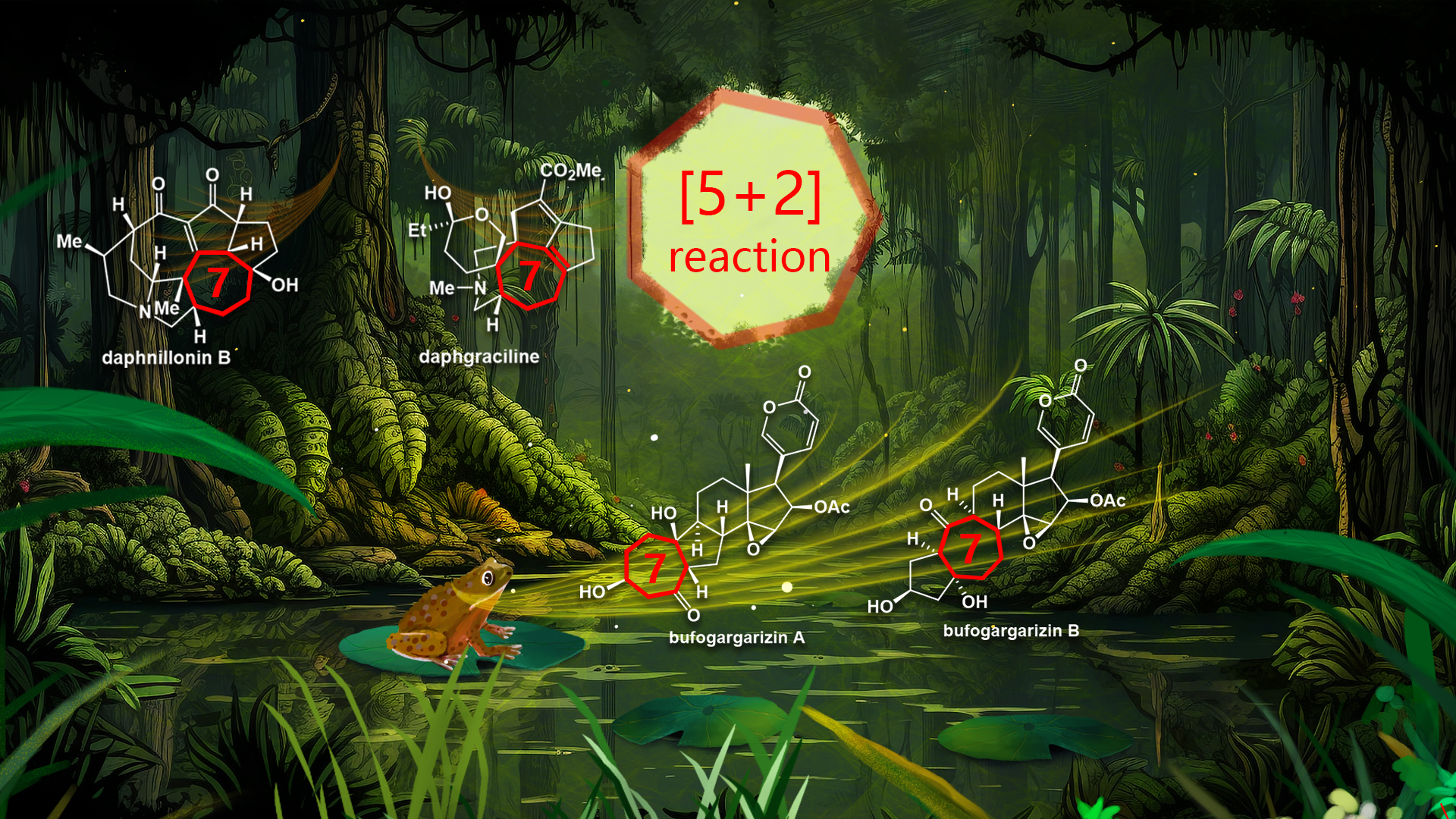
[5+2] New journey: the first total synthesis of the alkaloid Daphgraciline
Hao and co-workers isolated daphgraciline from the fruit of Daphniphyllum macropodum Miq. in 2008. Yuzurine-type alkaloid daphgraciline contains a compact [6−7−5−5−6] pentacyclic skeleton with a unique bridged azabicyclo[4.3.1] ring system and a spiro tetrahydropyran moiety. Daphgraciline has two vicinal all-carbon quaternary stereocenters at C5 and C8, and contains two sterically hindered tetrasubstituted double bonds (C9=C10 and C14=C15) and a C2 hemiketal moiety. The synthesis of daphgraciline, therefore, poses a formidable challenge.
Prof. Li’s group has achieved the first total synthesis of (±)-daphgraciline, A mild type II [5+2] cycloaddition-enabled diastereoselective and efficient synthesis of the unusual azabicyclo[4.3.1] ring system in yuzurine-type alkaloids. An IMDA reaction, followed by a benzilic acid-type rearrangement, allowed efficient construction of the compact tetracyclic skeleton of the yuzurine-type subfamily. The synthetically challenging spiro tetrahydropyran moiety was installed via a TiIII-mediated diastereoselective reductive epoxide coupling reaction.
This work is the first demonstration of using a type II [5+2] cycloaddition or TiIII-mediated reductive epoxide coupling in alkaloid synthesis. This method could be used to synthesize other members of the yuzurine-type subfamily of alkaloids and their analogs.
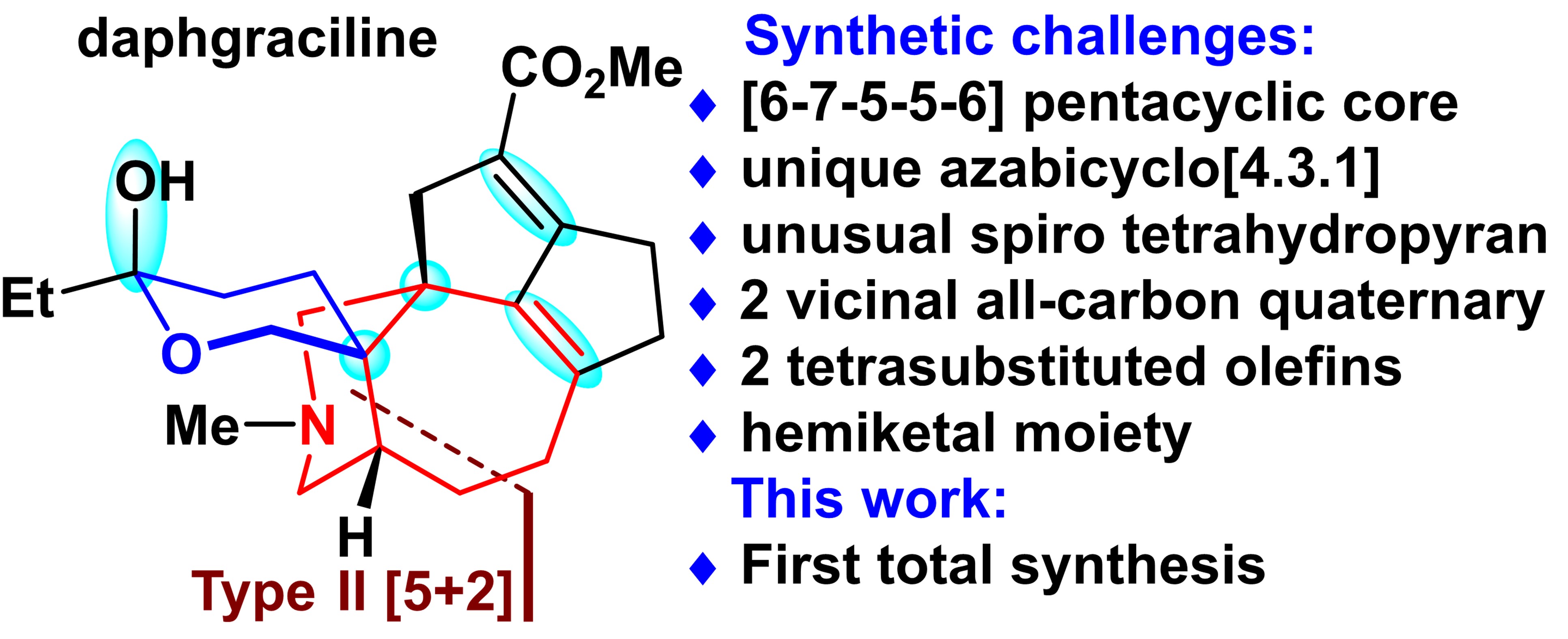
Figure 2. The first total synthesis of Daphgraciline. Image credit: JACS
Doctoral student Lixuan Li and Research Associate Professor Long Min are the co-first authors of this paper. Professor Chuang-Chuang Li is the sole corresponding author, and SUSTech is the only affiliated unit. Undergraduate Shuxiao Ji, Senior Research Scholar Chuang Qiao, and Research Assistant Peilin Tian also made important contributions to this work.
[5+2] New chapter: the first total synthesis of steroidal Bufogargarizins
Bufogargarizins A and B, two novel twin abeo-steroids with unprecedented skeletons, were isolated by Ye and co-workers in 2010 from the venom of Bufo bufo gargarizans. Structurally, twin bufogargarizins A and B contain an unusual [7−5−6−5] and [5−7−6−5] tetracyclic skeleton, respectively, and are the first examples of bufadienolides with a rearranged A/B ring system rather than the typical [6−6] skeleton. Furthermore, both bufogargarizins A and B possess a highly oxygenated core and have 10 stereocenters with an all-carbon quaternary center at C13. Therefore, bufogargarizins A and B pose considerable synthetic challenges.
Prof. Li’s team has achieved the first and asymmetric total synthesis of twin bufogargarizins A and B in 28 and 30 steps, respectively, from commercially available sitolactone. Remarkably, the synthetically challenging [7−5−6−5] tetracyclic core of bufogargarizin A was made efficiently by a unique intramolecular Ru-catalyzed [5 + 2] cycloaddition reaction. This work represents the first example of a transition metalcatalyzed [5 + 2] cycloaddition reaction of a vinyl ether cyclopropane-yne. In addition, the complex [5−7−6−5] tetracyclic core of bufogargarizin B was diastereoselectively reassembled from the [7−5−6−5] tetracyclic skeleton by the retro-aldol/transannular aldol cascade reactions.
This is the first example of a [7−5−X] skeleton reassembling to generate a [5−7−X] skeleton. The chemistry developed in this work will enable the diverse syntheses of other natural bufogargarizins, bufadienolides, and analogues that also have similar structural features to facilitate further biological research.
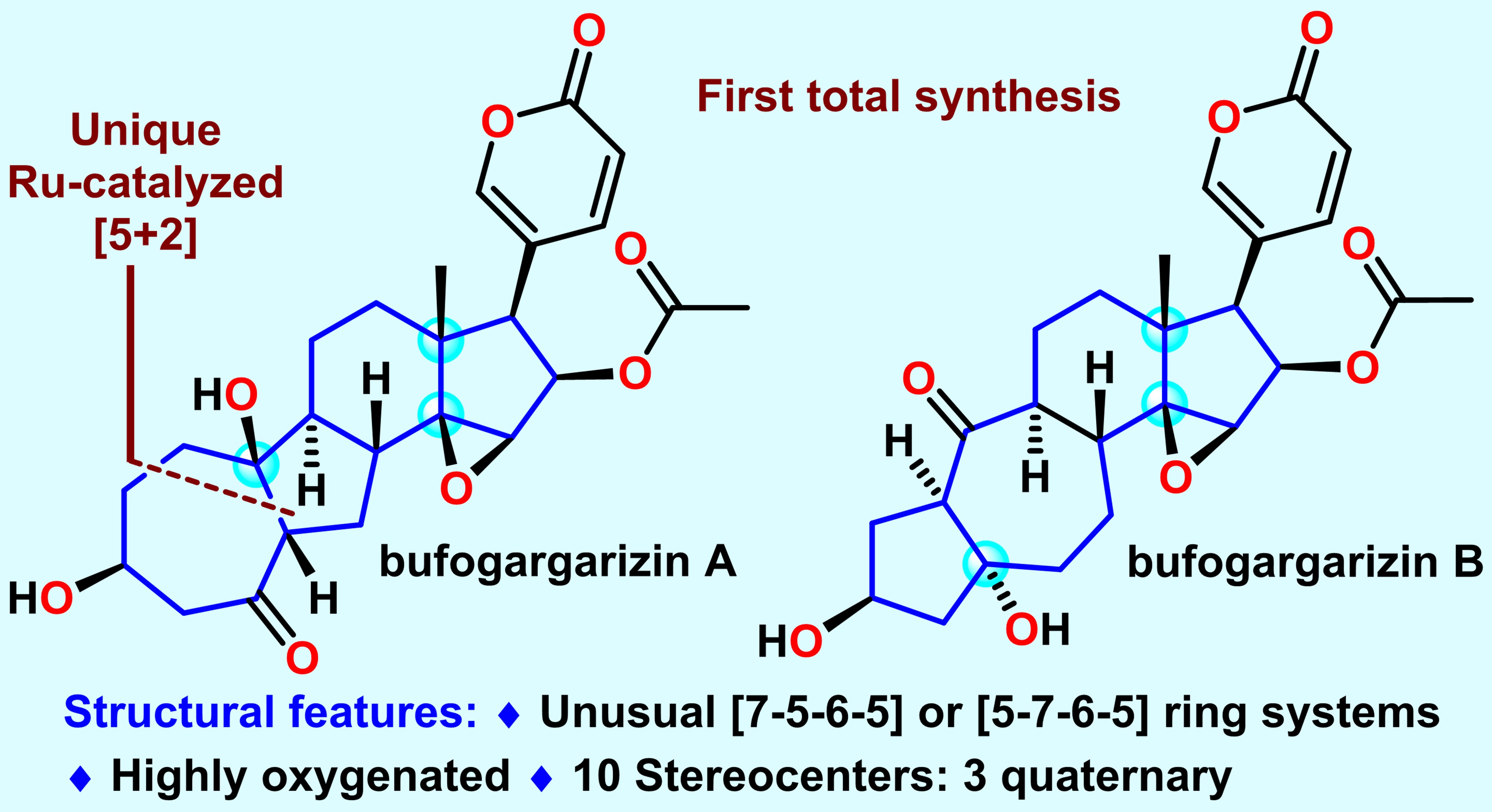
Figure 3. First total synthesis of Bufogargarizins A and B. Image credit: JACS
Doctoral student Liping Zhong is the first author of this paper. Professor Chuang-Chuang Li is the sole corresponding author, and SUSTech is the only affiliated unit. Postgraduate fellows Rui Feng and Jingjing Wang also made important contributions to this work.
These works were supported by the National Science Fund for Distinguished Young Scholars, Shenzhen Bay Laboratory, and Shenzhen Grabbs Research Institute.
Prof. Chuang-Chuang Li’s research group has been devoted to the development of innovative synthetic methods and the total synthesis of complex active natural products. By focusing on basic scientific issues in synthetic organic chemistry, the group has actively explored and developed original methodologies for the first total synthesis of complex active natural products.
Paper links (In order of appearance above):
JACS: https://pubs.acs.org/doi/10.1021/jacs.3c03755
JACS: https://pubs.acs.org/doi/10.1021/jacs.2c09548
JACS: https://pubs.acs.org/doi/10.1021/jacs.2c13494
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo By