CRISPR-Cas systems protect prokaryotes by conferring adaptive immunity against invading nucleic acids from viruses and plasmids. Immunity is acquired by integrating the invading nucleic acids between repeat sequences of the host CRISPR locus, which is then transcribed into precursor RNA (pre-crRNA). The pre-crRNA is processed into mature CRISPR-derived RNAs (crRNAs), which assemble with single- and multi-subunit Cas proteins to form crRNA-effector complexes responsible for the detection and subsequent degradation of the invading nucleic acids.
Depending on their genetic composition, CRISPR-Cas systems are categorized into six types (I-VI) in two major classes: class 1 systems (types I, III, and IV) consist of multi-subunit effector complexes, whereas class 2 systems (types II, V, and VI) are composed of single-subunit protein effectors; each of the types differs in its mechanism of action. Of the six types, the least understood is type IV, which can be further classified into five subtypes (IV-A to IV-E).
Distinct from the viral-targeting preference of other chromosomally encoded CRISPR-Cas types, type IV systems are primarily located on plasmids, with a strong bias toward targeting conjugative plasmids, suggesting a role in mediating plasmid–plasmid conflicts. In particular, type IV-A systems are found on large plasmids (>200 kb) and are significantly associated with antibiotic-resistance genes in multi-drug-resistant Klebsiella pneumoniae obtained from clinical samples, likely contributing to antibiotic-resistant plasmid stabilization while targeting other invasive plasmids. In contrast to the extensive studies describing viral targeting by chromosomally encoded CRISPR-Cas systems, however, the mechanisms underlying plasmid targeting by the plasmid-encoded type IV-A system remain unclear.
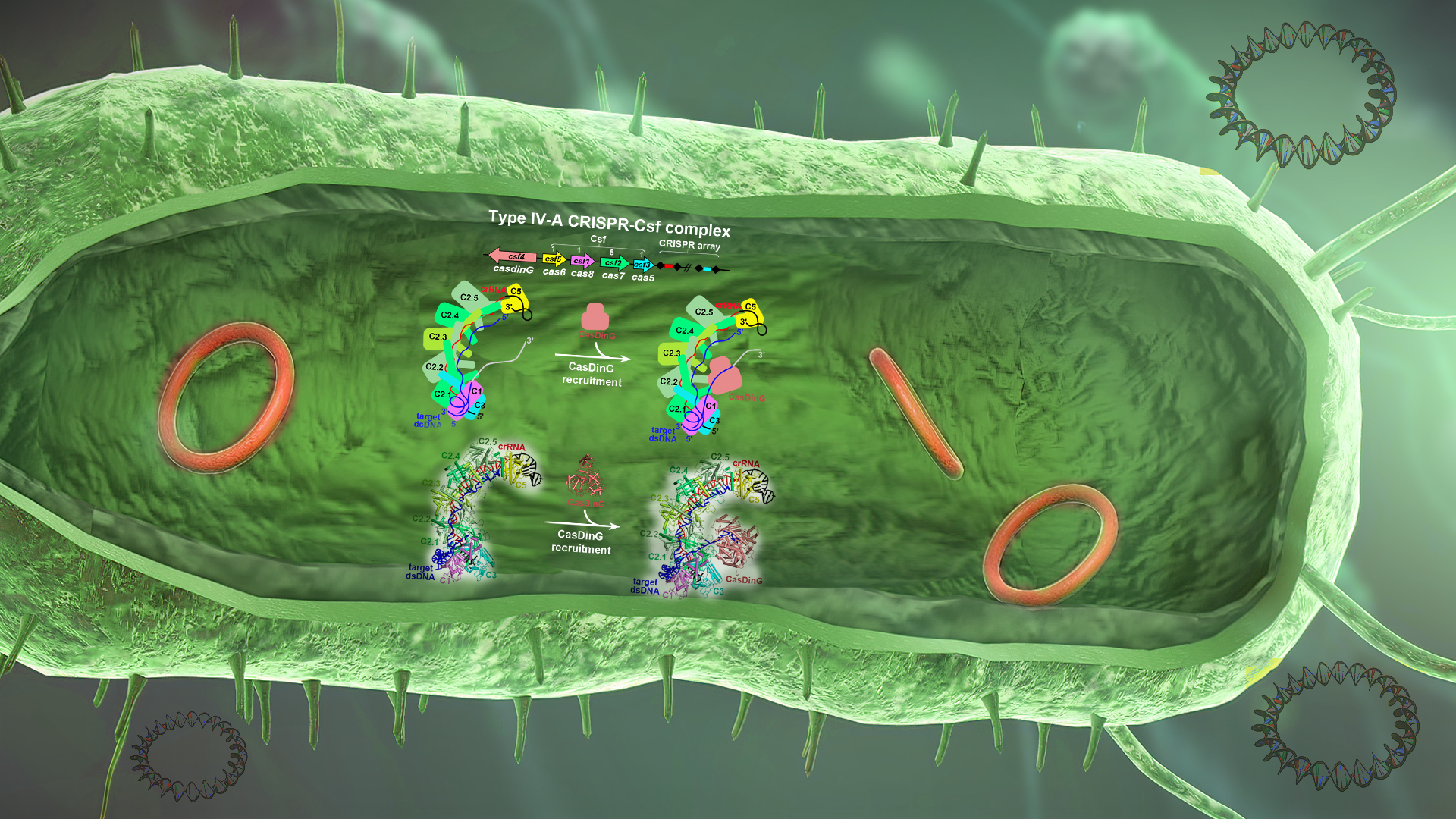
Associate Professor Ning Jia’s research team at the Southern University of Science and Technology (SUSTech), in collaboration with Associate Professor Hongda Huang’s team from the School of Life Sciences at SUSTech, have employed structural biology and biochemistry analyses to gain high-resolution insights into the type IV-A Csf complex. By examining various functional states, they provided a mechanistic understanding of the type IV-A Csf complex-mediated double-stranded DNA (dsDNA) targeting during the type IV-A CRISPR-Csf immunity.
Their research paper, entitled “Type IV-A CRISPR-Csf complex: assembly, dsDNA targeting, and CasDinG recruitment,” has been published in Molecular Cell.
The researchers provided high-resolution functional snapshots of type IV-A Csf complexes before and after target dsDNA binding, either in the absence or presence of CasDinG, revealing the mechanisms underlying CsfcrRNA complex assembly, ‘DWN’ PAM-dependent dsDNA-targeting, R-loop formation, and CasDinG recruitment (Figure 1). Furthermore, they established that CasDinG, a signature DinG family helicase, harbors ssDNA-stimulated ATPase activity and ATP-dependent 5′–3′ DNA helicase activity. They showed that CasDinG unwinds the nontarget strand (NTS) and target strand (TS) of target dsDNA from the CsfcrRNA complex. These molecular details advance our mechanistic understanding of type IV-A CRISPR-Csf function and should enable Csf complexes to be harnessed as genome-engineering tools for biotechnological applications.
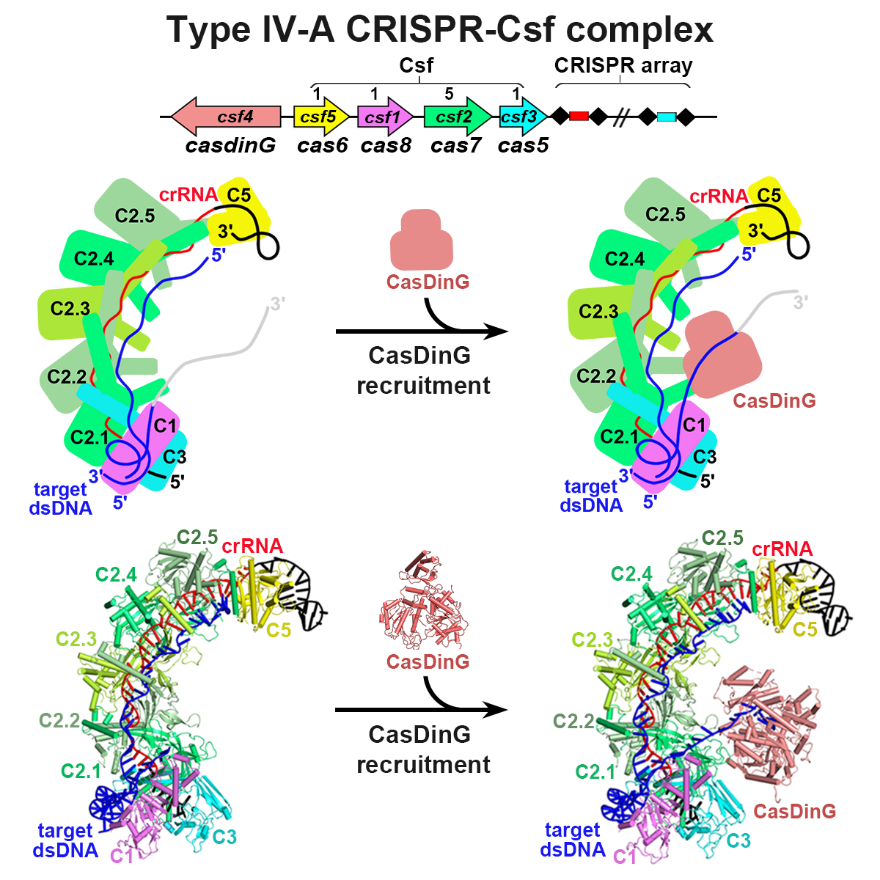
Figure 1. The assembly and target dsDNA recognition of the type IV-A CRISPR-Csf complex
Dr. Ning Cui and Dr. Jun-Tao Zhang are the co-first authors of this paper. Assoc. Prof. Ning Jia and Assoc. Prof. Hongda Huang at SUSTech are the corresponding authors.
The research was supported by the National Natural Science Foundation of China (NSFC), Guangdong and Shenzhen Natural Science Foundation, Key Project of Shenzhen Science and Technology Innovation Commission, Guangdong Provincial Science and Technology Innovation Council Grant, Shenzhen Science and Technology Program, and the Key Laboratory of Molecular Design for Plant Cell Factory of Guangdong Higher Education Institutes.
Paper link: https://doi.org/10.1016/j.molcel.2023.05.036
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo By