Sphingolipids are important membrane components and bioactive molecules in mammalian cells, which are involved in many important life activities. Sphingolipid levels in organisms need to be maintained in a balanced state, and too much or too little synthesis is harmful.
Dysregulation of sphingolipid homeostasis may lead to a variety of diseases, such as amyotrophic lateral sclerosis (ALS), cardiovascular diseases, and neurodegenerative diseases. The first step in the de novo synthesis of sphingolipids in mammals is catalyzed by the SPT complex on the endoplasmic reticulum. ORMDLs protein is a key negative regulator of SPT, which can form a stable complex with SPT and directly regulate the activity of SPT, thereby regulating the homeostasis of sphingolipids in cells.
Three ORMDLs subtypes, ORMDL1/2/3, exist in mammalian cells, and the human ORMDL3 gene is closely related to the susceptibility of childhood asthma. The SPT-ORMDL complex plays a crucial role in sphingolipid biosynthesis, homeostasis regulation, and various pathophysiological processes in mammals.
To elucidate the mechanism by which the SPT-ORMDL complex functions in mammals, Associate Professor Xin Gong’s research group from the School of Life Sciences at the Southern University of Science and Technology (SUSTech) has further studied the human SPT-ORMDL complex. The group has revealed that the human SPT-ORMDL complex senses the level of ceramide, an intermediate product of sphingolipid synthesis, thus negatively regulating the key mechanism of sphingolipid synthesis.
Their latest research results, entitled “Ceramide sensing by human SPT-ORMDL complex for establishing sphingolipid homeostasis”, were published in Nature Communications.
Prof. Gong’s team first obtained the protein of the human SPT-ORMDL complexes by optimizing in vitro recombinant expression and purification methods. They found that the activity of SPT-ORMDL can be inhibited by the important intermediate product ceramide in the sphingolipid biosynthesis pathway through in vitro enzyme activity experiments. To study the structural basis of ceramide regulation of SPT-ORMDL activity, they resolved the high-resolution three-dimensional structure of the human SPT-ORMDL3 complex bound to ceramide by using single-particle cryo-electron microscopy technology (Figure 1).
Through subsequent biochemical experiments, they discovered that ceramide binding plays an important role in the inhibition of SPT activity. It proved that ceramide binding can induce and stabilize the insertion of the N-terminus of ORMDL3 into the substrate binding pocket, thereby inhibiting the activity of SPT. Finally, they also proved through in vitro experiments that the SPT subunit mutations associated with juvenile ALS reported in recent years will lead to the inability of the SPT-ORMDL3 mutant protein to be effectively inhibited by ceramide.
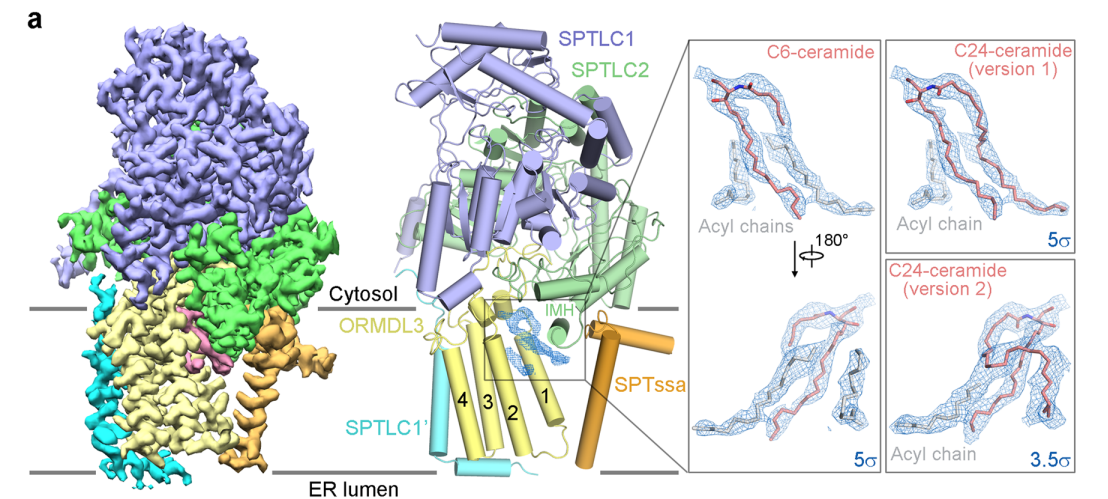
Figure 1. Overall structure of human SPT-ORMDL3 complex bound to ceramide
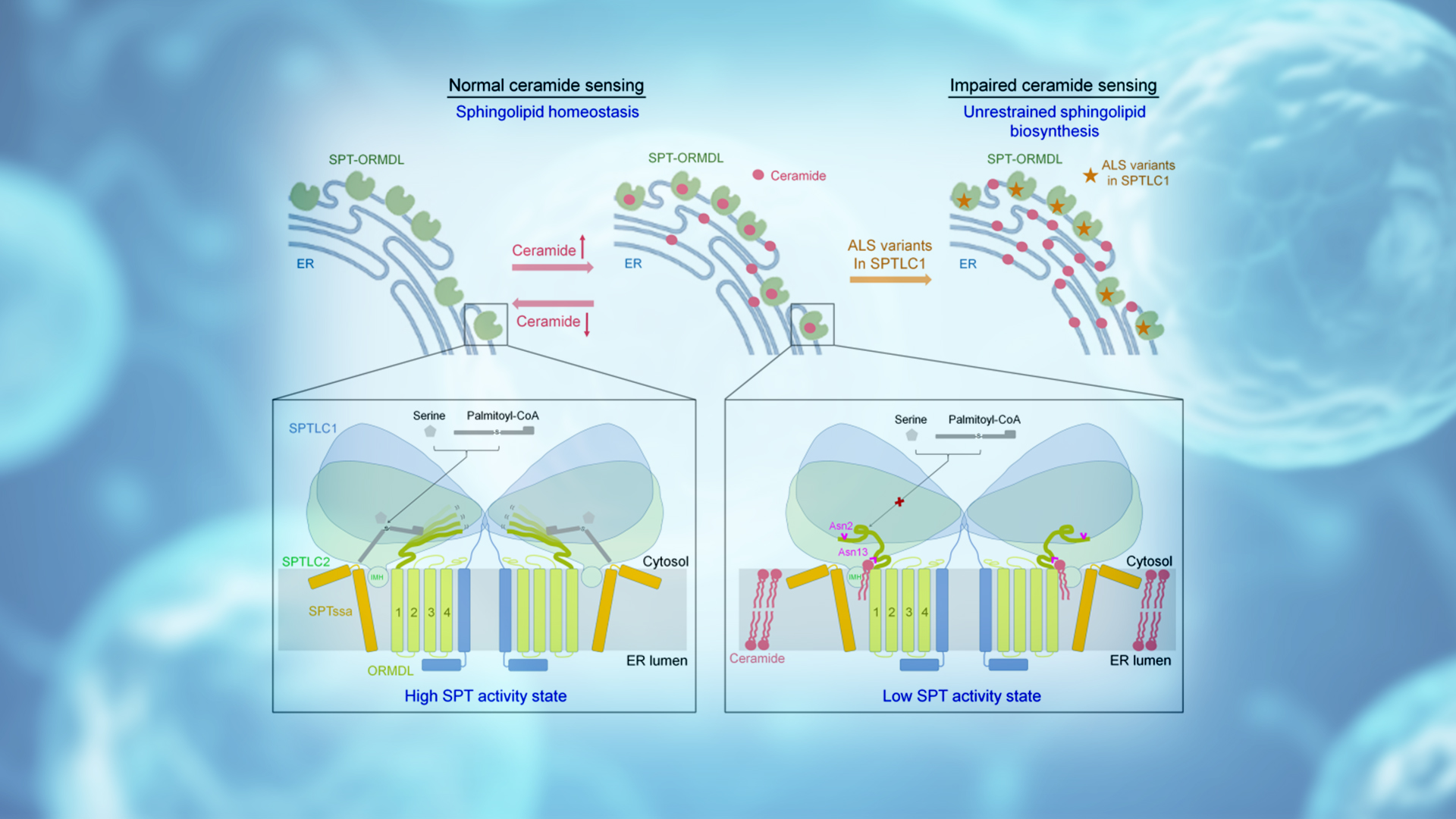
Based on the structural and biochemical experimental results in this study, the researchers finally proposed a working model in which ceramide participates in the homeostatic regulation of the SPT-ORMDL complex (Figure 2). In this model, the SPT-ORMDL complex acts as a ceramide sensor on the endoplasmic reticulum membrane to sense ceramide levels in cells.
When the ceramide level is relatively low, the N-terminal of ORMDL is highly flexible, which keeps the SPT-ORMDL complex in an active state, thereby increasing the synthesis of sphingolipids. As ceramide levels increase, ceramide binding to the SPT-ORMDL complex induces and stabilizes the N-terminal of ORMDL into an inhibitory conformation that prevents substrate entry, converting the complex into an inactive state, reducing sphingolipid synthesis, and maintaining sphingolipid homeostasis in cells. The researchers also noted that ALS-associated SPT mutants cannot normally sense ceramide levels, resulting in unregulated SPT activity and unrestricted biological sphingolipid synthesis.
These results suggest that the SPT-ORMDL complex acts as a ceramide sensor that regulates sphingolipid homeostasis in mammals and is essential for the health of the organism. In addition, the sensing mechanism proposed in this study also provides an important reference for the homeostatic regulation of other biosynthetic pathways in organisms.
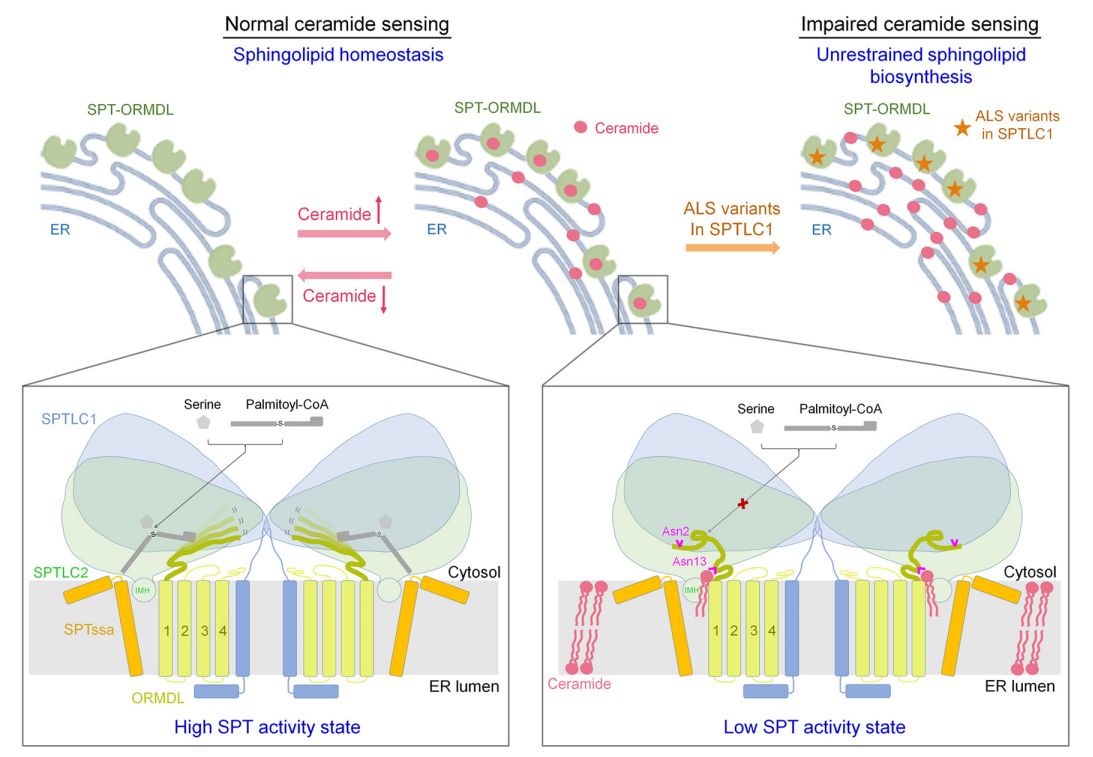
Figure 2. A working model for homeostatic regulation of sphingolipid synthesis in mammals
Prof. Xin Gong from SUSTech and Prof. Binks W. Wattenberg from Virginia Commonwealth University are the co-corresponding authors of this paper. Tian Xie and Peng Liu, members of Prof. Gong’s research team, contributed equally to this study.
This research was supported by the National Natural Science Foundation of China (NSFC), Department of Science and Technology of Guangdong Province, and the Shenzhen Science and Technology Innovation Commission. The cryo-electron microscopy data collection and processing for this work was supported by the Cryo-Electron Microscopy Center of SUSTech, and the mass spectrometry experiments by the Public Analysis and Testing Center of SUSTech.
Paper link: https://www.nature.com/articles/s41467-023-39274-y
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo By