Epstein-Barr virus (EBV) is a global public health concern, as it is known to cause multiple diseases while also being etiologically associated with a wide range of epithelial and lymphoid malignancies. It holds the distinction of being the first human oncogenic virus. This pathogen is responsible for causing infectious mononucleosis (IM) and has strong associations with various autoimmune diseases, including nasopharyngeal carcinoma, certain gastric cancers, various lymphomas, and multiple sclerosis. Despite this virus’s considerable impact, there is currently no available prophylactic vaccine against EBV.
EBV features numerous viral glycoproteins on its membrane, which facilitates virus entry into host cells by binding to their corresponding receptors. One of the pivotal glycoproteins, gB, serves as the key fusion protein for the virus. Gleaning signals from other viral glycoproteins, gB binds to the viral receptor on the host cell membrane, thus initiating the fusion of the virus with the cell membrane. While gB presents itself as an attractive candidate for an Epstein-Barr virus vaccine, several limitations impede its broader application in this capacity. These constraints include a modest humoral immune activation effect, a relatively short duration of immune response, and an inadequate ability to induce neutralizing antibodies.
Professor Zheng Liu from the Cryo-Electron Microscopy Center at the Southern University of Science and Technology (SUSTech), in collaboration with Professors Musheng Zeng and Qian Zhong from the Sun Yat-sen University Cancer Center (SYSUCC), has recently published a study that has focused on the assembly of a nanoparticle vaccine containing gB, achieved through meticulous structural design and in vitro expression. The results demonstrated not only the excellent antigenicity and stability of the vaccine but also its capacity to effectively stimulate the production of neutralizing antibodies against EBV. This groundbreaking work serves as a crucial milestone for future research and development in EBV vaccines.
Their research work, entitled “A gB Nanoparticle Vaccine Elicits a Protective Neutralizing Antibody Response against EBV,” has been published in Cell Host & Microbe.
In the study, the researchers employed computer-assisted docking to calculate the optimal link distance between gB and various nanoparticle skeleton proteins. Based on this computational model, they designed the nanoparticle gB-I53-50 NP, which was subsequently assembled in vitro. This assembly was rigorously verified through a combination of biochemical and structural biology techniques, conclusively confirming that the nanoparticle effectively expressed and presented the gB protein of the Epstein-Barr virus on the surface of the vaccine.
Subsequently, they conducted an array of assays, including protein intrinsic fluorescence scanning and biofilm interference, to elucidate that the vaccine enhanced the antigenicity of the gB antigen. Furthermore, they observed that the nanoparticles displayed commendable stability, both at 4°C and room temperature. This stability aspect is pivotal, as it forms a solid foundation for the preparation and transportation of the vaccine.
Using cutting-edge techniques such as single-particle cryo-electron microscopy and cryo-electron tomography, the researchers embarked on a comprehensive structural analysis of the gB-I53-50 nanoparticles. This analysis determined a high-resolution structure that not only confirmed the structural integrity of the gB-I53-50 nanoparticles, but also highlighted the unaltered structural integrity of the gB protein adorning the nanoparticle surface. Additionally, the study shed light on the remarkable flexibility of the protein’s swing, further enhancing our understanding of its functional dynamics.
Through a series of mouse and non-human primate immune experiments, they made groundbreaking discoveries. They found that the vaccine had the remarkable ability to induce neutralizing antibodies more efficiently against EBV when compared to traditional subunit protein vaccines. The vaccine simultaneously demonstrated the capacity to inhibit EBV infection in both epithelial and B cells, while also eliciting a stronger T-cell activation response. Furthermore, employing a humanized mouse model, the researchers established that the serum polyclonal antibodies induced by the gB nanoparticle vaccine in primates had a significant protective effect on humanized mice against EBV infection and lymphoma development. Impressively, this protective effect endured for a substantial ten weeks after immunization in primates, affirming the vaccine’s capability to induce robust and enduring neutralizing protection. As a result, this nanoparticle protein vaccine emerges as a highly promising next-generation candidate for an EBV vaccine, possessing significant potential for clinical application. It offers a valuable blueprint for the development of vaccines against other pathogenic herpes viruses.
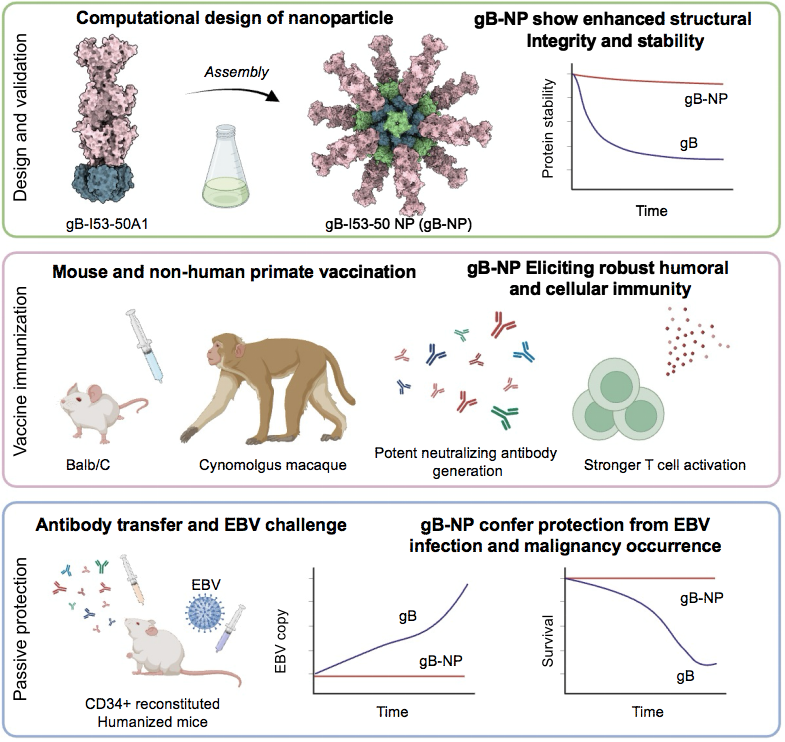
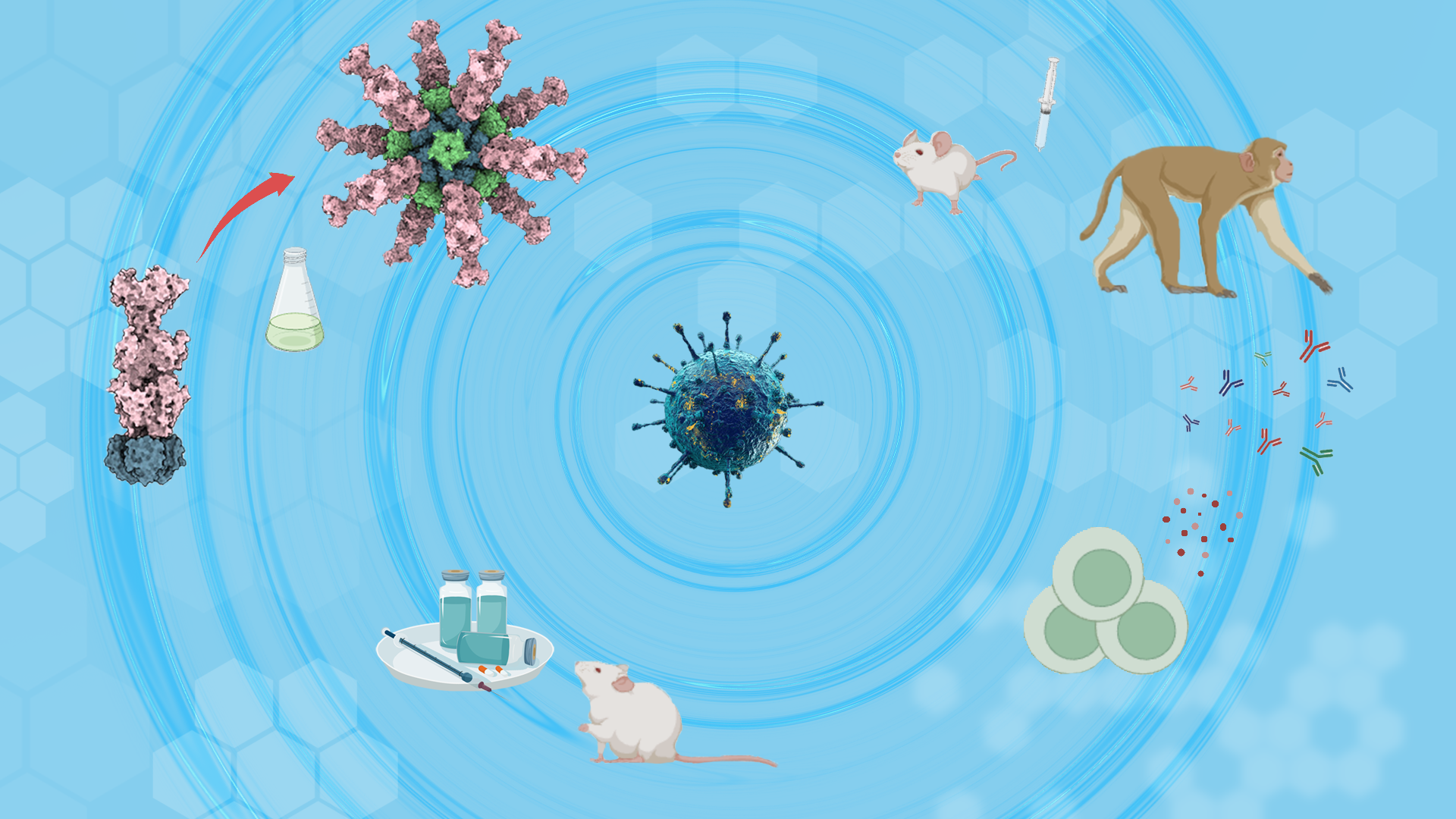
Figure 1. An effective EBV vaccine is urgently needed but remains elusive. Sun et al. designed a gB nanoparticle vaccine with enhanced stability and immunogenicity that elicits robust and durable protective antibodies in preclinical models. Immunization of humanized mice prevents lethal EBV infection, thereby offering a potential strategy for EBV vaccine development.
Drs. Cong Sun, Yinfeng Kang, and Yina Liu from SYSUCC, and Ph.D. candidate Xinyan Fang from the School of Life Sciences at SUSTech, are the co-first authors of this paper. Prof. Zheng Liu from SUSTech and Profs. Musheng Zeng and Qian Zhong from SYSUCC are the co-corresponding authors.
The research was supported by the National Natural Science Foundation of China (NSFC) and the National Key Research and Development Plan.
Paper link: https://www.cell.com/cell-host-microbe/fulltext/S1931-3128(23)00378-5
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo By