Recently, Associate Professor Cong YU, Associate Professor Zhiyi WEI, Professor Chuanyue WU and Associate Professor Yi DENG, from the Department of Biology, published a new study entitled, Structural basis of kindlin-mediated integrin recognition and activation , in the Proceedings of the National Academy of Sciences of the United States of America, an international top journal. Huadong LI, the research assistant of the Department of from Associate Professor Yu’s group is the first author, and Yi DENG as the co-first authors. Cong YU, Zhiyi Wei and Chuanyue Wu are the co-corresponding authors. Jirong LIN who graduated in 2017 at SUSTech was involved in the research as well.
Their study focus on kindlin protein which are integrin-binding proteins involved in integrin activation, an essential process for many fundamental cellular activities including cell-matrix adhesion, migration, and proliferation.
Mutations of kindlins lead to many diseases, such as Kindler syndrome, associated with skin blistering and atrophy; leukocyte adhesion deficiency; and cancers. However, the molecular basis underlying kindlin-mediated integrin activation remains to be determined. In this paper, they report the structural basis of the specific interaction between kindlins and integrins. Furthermore, they demonstrate that kindlins synergize integrin activation by forming a dimer, providing a new model for understanding integrin signaling. Based on the structural information, they also interpret disease-causing mutations found in kindlins at the atomic level, which can be useful for understanding and treating these diseases.
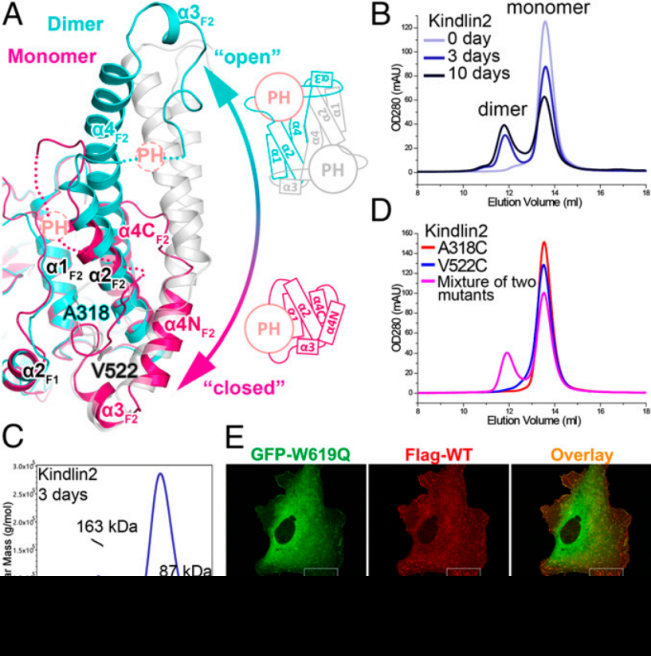
FIG: On the left, A: schematic diagram of kindlin; B: kindlin structure. On the right, A; the structure comparison of kindlin monomer anddimer; B-E, in vitro and in vivo experiments verified the dimer-formation of kindlin
This research has combined the structural biology, biochemistry and cell biology to solve the long-standing problem in the related field. The structural basis of the interaction between kindlin and integrin is systematically revealed, and the three-dimensional structures also helps to provide the informations to understand the kindlin mutations in many genetic diseases. Richard Hynes, an editor of the American Academy of Sciences and an expert in integrin research, invited Professor Reinhard Fässler of the Max Planck Institute in Germany to write a special comment for this article. The laboratory will continue to study the important issues related to the field.
This study was supported by the Research Center of Life Science of SUSTech, the National Center for Protein Science Shanghai , Shanghai Synchrotron Radiation Facility, the National Natural Science Fund Committee, the National Technological Project, the Guangdong Province Natural Science Fund Committee, the Shenzhen Commission on Innovation and Technology and SUSTech Fund.
Proofread ByJeremy Welburn
Photo ByDepartment of Biology