Anxiety disorder is one of the common mental illnesses. According to large-scale population surveys, up to 33.7% of people have suffered from anxiety disorder in their lifetime. Inflammation is one of the key mechanisms of stress-induced anxiety disorder, and both cytokine intervention and anti-inflammatory drug treatments can improve anxiety-like behavior in rodents. Microglia are the primary immune cells in the brain, playing a central role in neuroinflammation, immune surveillance, and maintaining brain homeostasis. Therefore, anti-inflammatory therapies targeting microglia could be one of the effective strategies for the clinical treatment of anxiety disorders.
Na+/K+-ATPase (NKA) plays a crucial role in maintaining the electrochemical gradient on the cell membrane by transporting three Na+ out of the cell and two K+ into the cell. NKA also participates in Ca2+ signal transduction and neurotransmitter release by coordinating ion concentration gradients on the cell membrane. Simultaneously, NKA can cooperate with multiple ion channels on the cell membrane to form a dynamic network of ion homeostasis regulation and influence cell communication by regulating chemical signals and ion balance between different types of cells. However, NKA is not just a simple ion pump, but also a signaling transduction protein or cell protection protein.
Professor Jinsong Bian’s research group from the Department of Pharmacology, School of Medicine at the Southern University of Science and Technology (SUSTech) has long focused on the physiological functions of NKA and its role in disease development. The group recently published a study that revealed a new anti-inflammatory function of NKA (also known as the sodium-potassium pump) and proposed a new strategy to treat stress-induced anxiety disorder by targeting NKA. In 2021, the group first reported in Science Advances that the loss of NKA on dopaminergic neurons is an important mechanism for the onset of Parkinson’s disease. This study has once again found the important role of NKA on microglia in the development of anxiety disorder.
Their paper, entitled “Disruption of the Na+/K+-ATPase-purinergic P2X7 receptor complex in microglia promotes stress-induced anxiety”, has been published in Immunity, a top journal by Cell Press covering the most important advances in immunology.
Through various behavioral experiments such as the Open Field Test and Elevated Plus Maze test, the researchers found that mice with global knockdown of NKAα1 showed markedly severe anxiety-like symptoms in both restraint stress and foot-shock models. By injecting the AAV virus to create mice with NKAα1 specifically knocked down in the ventral hippocampus (vHIP), and by creating mice with specific knockout of NKAα1 in microglia, they found that stress-induced anxiety-like symptoms in mice were also significantly exacerbated. Further biochemical experiments testing neuroinflammation in the vHIP revealed that further activation of microglia led to hyperexcitability of neurons, thus exacerbating anxiety phenotype. These results suggest that NKAα1 on microglia in the vHIP has a significant impact on anxiety.
To explore the mechanism of NKAα1-mediated anti-neuroinflammation, Professor Jinsong’s group used in situ hybridization experiments, immunoprecipitation technique, and primary neurons, astrocytes, and microglia cultured in vitro. They found that NKAα1 interacts with a purinergic receptor, P2X7R. By using Surface Plasmon Resonance (SPR) techniques, they confirmed the existence of an NKAα1-P2X7R complex. Through this complex, NKAα1 effectively reduced P2X7R-mediated K+ extrusion, resisting the activation of microglia. However, under stress conditions, the membrane expression of NKAα1 is lost, promoting K+ extrusion mediated by free-form P2X7R, exacerbating the release of inflammation factor IL-1β.
Based on the above pathological mechanisms, the group developed a monoclonal antibody targeting the extracellular segment of NKAα1. This antibody stabilizes the expression of NKAα1 on the cell membrane, thereby preventing the occurrence of neuroinflammation, reducing the excitability of neurons in the vHIP, and achieving the effect of treating anxiety-like behavior in mice.
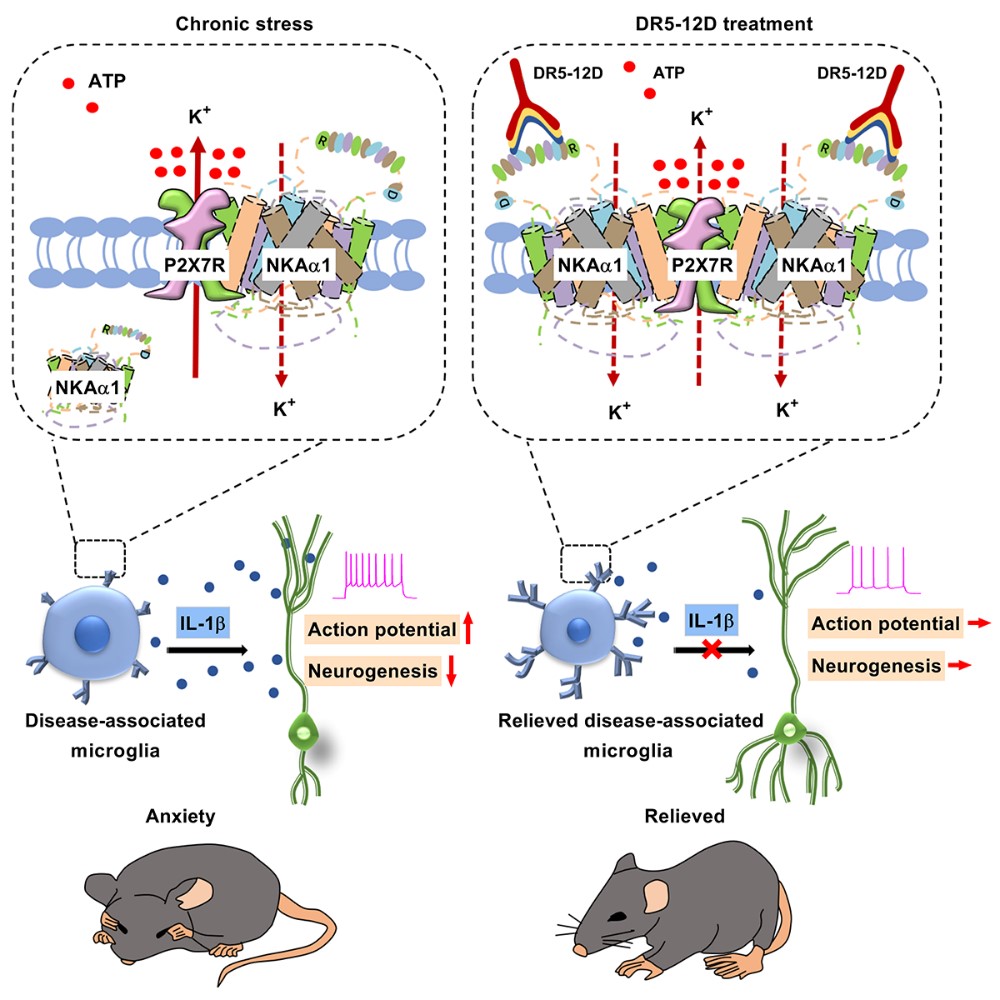
Figure 1. Stress-induced reduction in NKAα1 membrane expression activates microglia and releases IL-1β, exacerbating anxiety.
This research not only reveals how non-neuronal cells in the brain coordinate neuronal function in health and disease, but also provides a potential new target for the treatment of anxiety. At the same time, it provides a valuable new direction for the clinical diagnosis and treatment of stress-related mental disorders. Based on these important discoveries, the journal Immunity has published a preview together with this research article, considering that the study had made significant academic contributions and conceptual breakthroughs in understanding the mechanisms of stress-induced anxiety, especially how microglia are involved in the onset of mental disorders.
Songqiang Huang, a doctoral student from the School of Medicine at SUSTech, is the first author of the paper. Professor Jinsong Bian from the School of Medicine at SUSTech and Associate Professor Xiaowei Nie from Shenzhen People’s Hospital are the corresponding authors. Other contributors to this study include master’s student Xiaoqian Lin, doctoral student Kangtai Xu, and undergraduate student Kun Li.
The research was supported by the National Natural Science Foundation of China (NSFC), Shenzhen Outstanding Youth Fund, and the Shenzhen Science and Technology Innovation Commission.
Paper link: https://www.cell.com/immunity/abstract/S1074-7613(24)00044-X
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo ByDepartment of Pharmacology