Glioblastoma multiforme (GBM) is the most common and lethal brain tumor with an extremely poor prognosis. The main cellular components of the bulk consist of heterogeneous glioblastoma stem cells (GSC) and their differentiated counterparts. Current therapies for GBM inevitably harm normal cells, causing a variety of severe complications. Therefore, therapies with better precisions based on variations among GSC, differentiated glioblastoma cells, and normal cells in the tumor and its microenvironment are urgently needed.
Mitochondria are essential in GBM and serve as therapeutic targets. Mounting evidence demonstrates mitochondria are genetically altered, functionally dysregulated, and metabolically reprogrammed in GBM. However, reliable direct evidence from a structural point of view is largely missing.
The research team led by Professor Qing-Tao Shen from the School of Life Sciences at the Southern University of Science and Technology (SUSTech) has recently identified novel inter-mitochondrial structural signatures from glioblastoma cells and proved their diagnostic potential through predictive modeling.
Their work, entitled “Dysregulated inter-mitochondrial crosstalk in glioblastoma cells revealed by in situ cryo-electron tomography”, has been published in the Proceedings of the National Academy of Sciences (PNAS), a prestigious high-impact journal that covers biological, physical, and social sciences.
In this study, the researchers utilized in situ cryo-electron tomography (cryo-ET) to quantitatively investigate nanoscale details of hundreds of mitochondria from six different cell types directly within their native cellular context of glioblastoma. To overcome the limitations of the small field of view and biased sampling often associated with cellular cryo-ET data collection, they adopted a random data acquisition scheme to obtain less biased and more comprehensive knowledge.
The subsequent quantitative analyses show substantial variations in inter-mitochondrial crosstalk patterns between glioblastoma cells and cancer-free controls. Specifically, glioblastoma cells own markedly more inter-mitochondrial junctions (IMJ) of several types for communications compared to cancer-free astrocytes and microglia cells. Intriguingly, nanotunnel-like bridges are identified in the glioblastoma cells as a newly discovered inter-mitochondrial structure.
Based on the quantifications, the researchers explored the potential mechanism associated with mitochondrial nanotunnels. Detailed analyses show that the mitochondrial nanotunnels found in GBM strongly correlate with the presence of microtubule pile-ups in the mitochondrial vicinity, suggesting a microtubule-dependent mechanism for its formation (Figure 1). They also performed predictive modeling to distinguish glioblastoma cells from cancer-free controls with the quantified inter-mitochondrial crosstalk information, plus other mitochondria-organelle and intra-mitochondrial features. The prediction accuracy of the nonlinear modeling is above 95%.
Although functional explanations of the observed structural abnormalities require further substantiation, this work demonstrates the potential of quantitative in situ cryo-ET as a biomarker technology to derive models of mitochondrial interactome in their native cellular context, providing clues to therapeutics and diagnosis of glioblastoma and other mitochondria-related diseases.
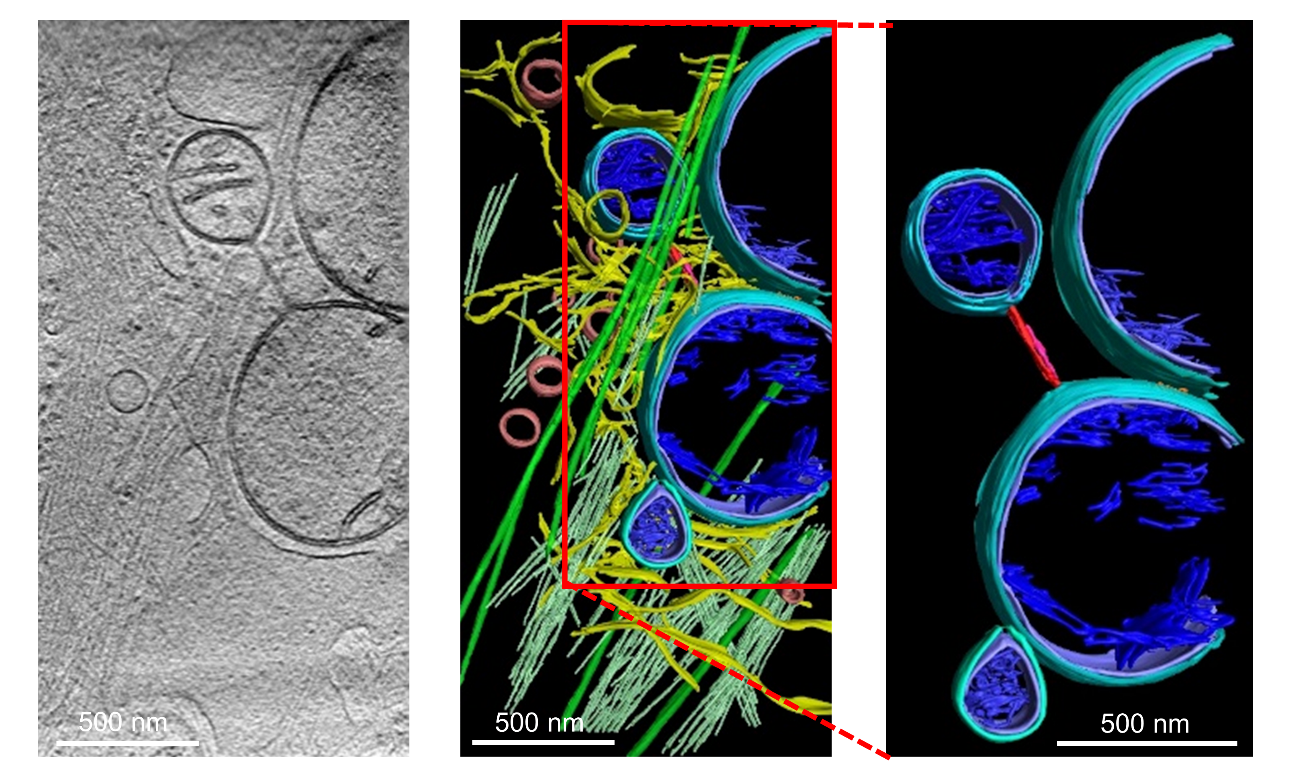
Figure 1. Microtubule-dependent Mitochondrial Nanotunnels in GBM
Research Associate Professor Rui Wang and Ph.D. student Huan Lei from the School of Life Sciences at SUSTech are the co-first authors of this paper. Associate Professor Qing-Tao Shen is the corresponding author.
This work was supported by the National Key Research and Development Program of China, National Natural Science Foundation of China (NSFC), and the Shanghai Basic Research Program. The cryo-electron microscopy data collection and processing for this work was supported by the Cryo-Electron Microscopy Center of SUSTech and the CryoEM facility for Marine Biology at the Qingdao National Laboratory for Marine Science and Technology (QNLM).
Paper link: https://doi.org/10.1073/pnas.2311160121
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo BySchool of Life Sciences