Photosynthesis converts light energy into chemical energy and stores it in organic molecules in organisms. Chlorophyll-driven aerobic photosynthesis mainly occurs in plants, algae, and cyanobacteria. Cyanobacteria, as prokaryotes, are the only bacteria that carry out oxygen-evolving photosynthesis. They can inhabit different environments, including the ocean, freshwater, and land, and also include some extreme environmental conditions. Chlorophyll a (Chl a) is the main light-harvesting pigment in the photosystem of cyanobacteria. Most cyanobacteria use phycobilisomes as the main part of the light-harvesting antenna system. In all photosynthetic species, including algae and higher plants, the main pigment is Chl a. In green algae and higher plants, the light-harvesting antennas contain both Chl a and Chl b.
In 1996, it was found that Acaryochloris Marina (A. marina) contained water-soluble phycobilisomes and Pcb antennas in the membrane, and Chl d was the main pigment. Chl d differs from Chl a in that it has a formyl group instead of a vinyl group at the third position. This structural change leads to a red shift of the maximum long wavelength absorption of pigment about 30 nm. With the help of red-shifted Chl d, A. marina can extend its photosynthetic activity spectrum region to a long wavelength region above 700 nm, thus strengthening its adaptability to a low light environment and promoting photoautotrophic growth.
Unlike other cyanobacteria, A. marina contains Chl d-binding light-harvesting antennas, named Pcb, which are encoded by pcbA and pcbC. These Pcb proteins differ from the intra-membrane light-harvesting antennas of higher plants and green algae. The molecular weight of the Pcb protein is about 35 kDa, which is homologous to the CP43 of PSII and IsiA protein induced by some cyanobacteria during iron deficiency. The Pcb protein typically occurs in cyanobacteria such as Prochlorothrix. It binds to both Chl a and Chl b, serving as peripheral light-harvesting antennae for both PSI and PSII. A. marina contains both Pcb light-harvesting protein in the membrane and phycobiliprotein outside the membrane, which can capture light energy and transfer it to the PSII core.
But up to now, the Pcb subunit of this supercomplex, the interaction between the Pcb subunit and PSII core, and the energy transfer pathway between pigments are still unknown. At the same time, the molecular mechanism of photosystem II (PSII) utilizing far-red light is still unclear.
Professor Peiyi Wang from the Cryo-EM Center at the Southern University of Science and Technology (SUSTech), in collaboration with Professor Guangye Han from the Institute of Botany, Chinese Academy of Sciences (IB-CAS) and Professor Xing Zhang from Zhejiang University, have resolved the structure of A. marina photosystem II-Pcb tetrameric supercomplex using cryo-electron microscopy (Fig 1).
Their results, entitled “Structure of a unique PSII-Pcb tetrameric megacomplex in a chlorophyll d-containing cyanobacterium”, have been published in Science Advances, a multidisciplinary journal covering all areas of science.
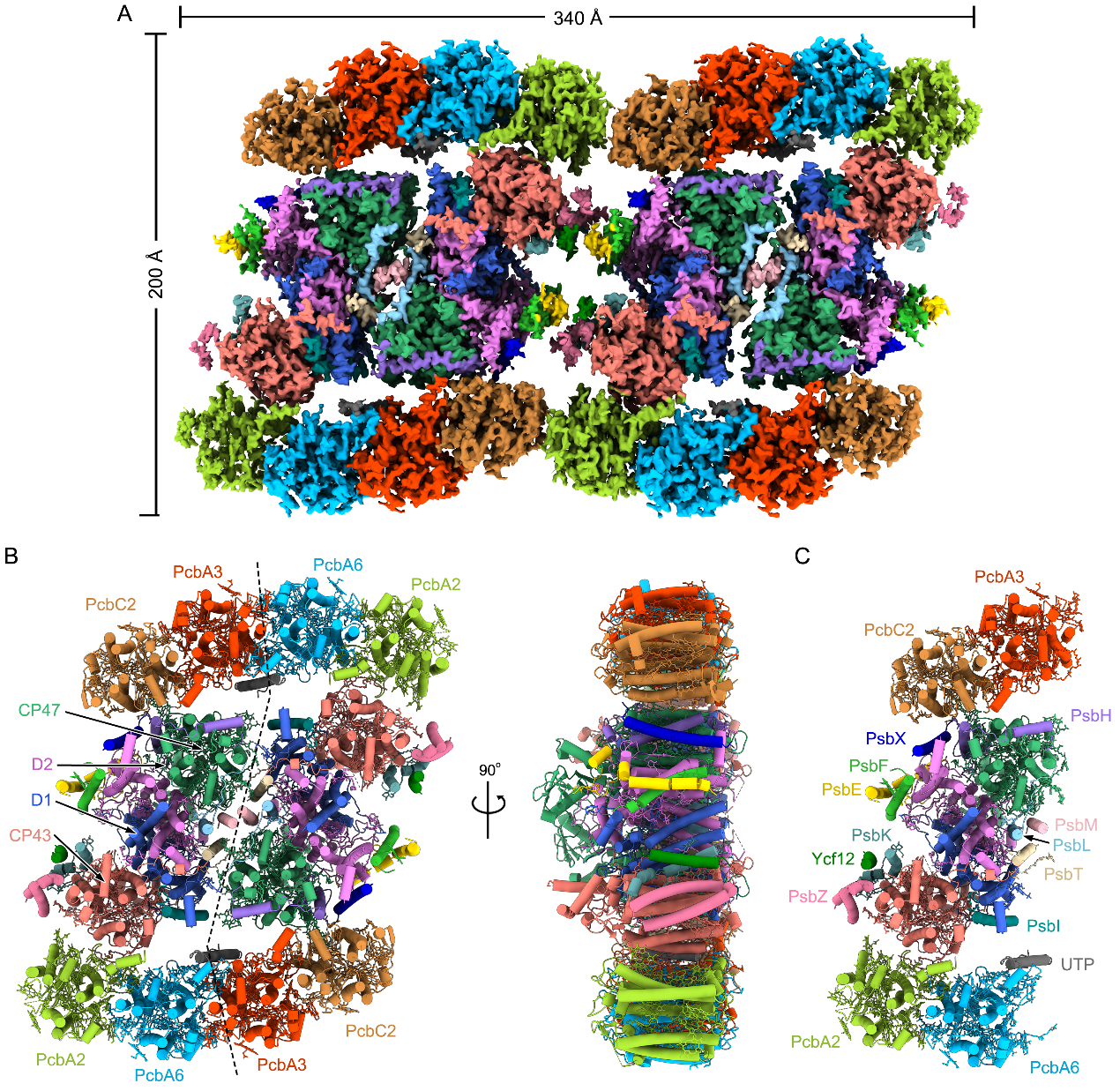
Figure 1. Overall structure of the PSII-Pcb tetramer from A. marina.
The PSII-Pcb megacomplex is a tetramer with dimensions of ~340 Å in length, 200 Å in width, and 90 Å in height. Two PSII-Pcb dimers are arranged side by side and associated with 16 Pcb antennas. In total, 80 subunits and 624 cofactors were identified in the whole PSII-tetramer of A. marina, which gives rise to a total molecular weight of ~1.9 MDa. Each PSII monomer contains 15 core subunits and four Pcb antenna subunits (named PcbA2, PcbA6, PcbA3, and PcbC2 (Fig 1). Among these Pcb subunits, PcbA2 and PcbA6 bind directly to the CP43 side of the PSII core, whereas PcbA3 and PcbC2 are attached to the CP47 side through PsbH. Based on the unique structural characteristics of the complex and the arrangement of pigment molecules, the researchers identified numerous pathways for capturing and transmitting light energy within this complex (Fig 2).
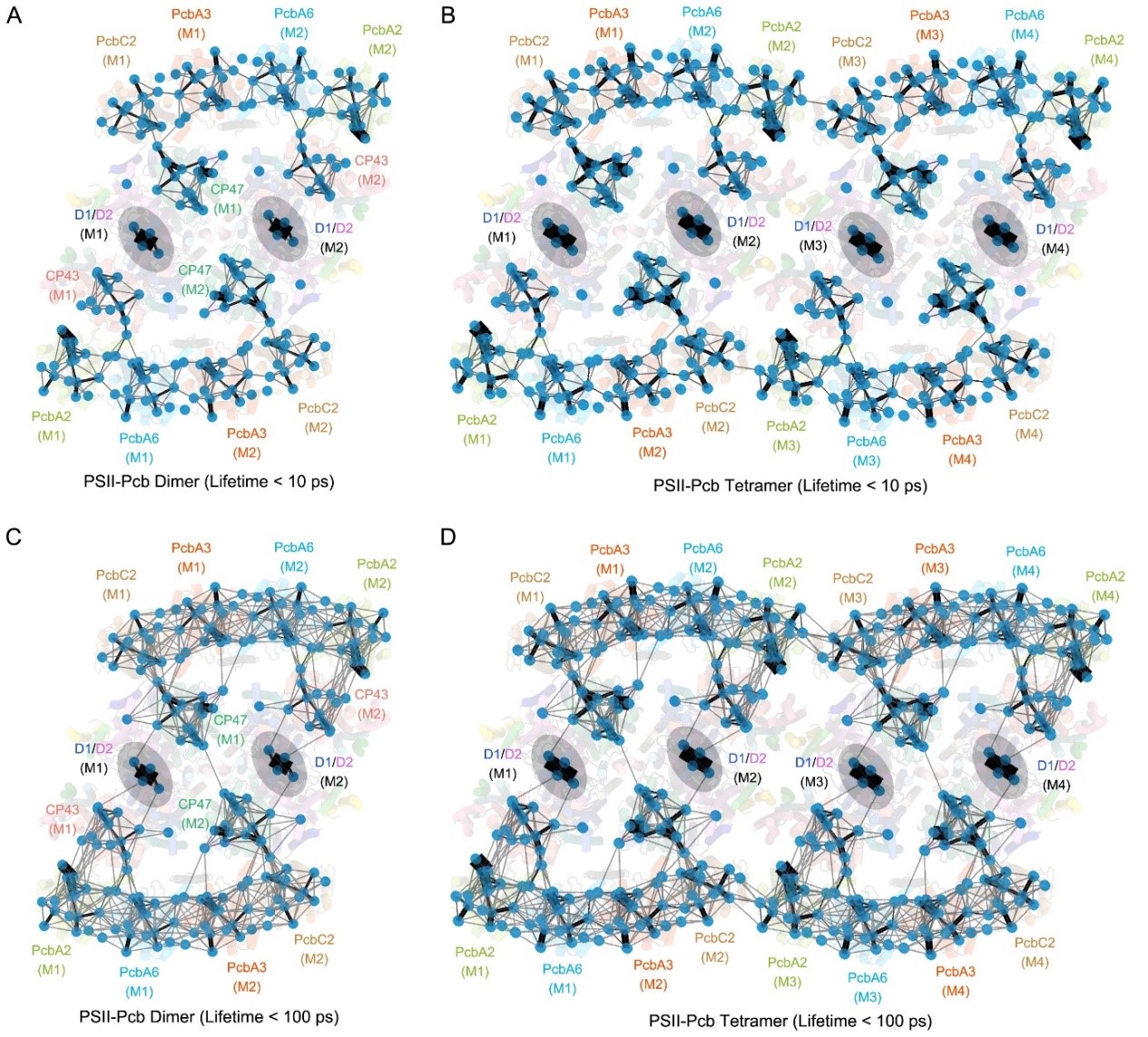
Figure 2. FRET networks within the PSII-Pcb dimer and tetramer complex from A. marina
One of the distinct features of the PSII-Pcb tetramer is the binding of transmembrane Pcb proteins as the major light-harvesting protein. The Chl d-binding Pcb protein is a unique LHC protein found in A. marina, which may be similar to the Pcb proteins found in Chl b containing cyanobacteria like Prochlorothrix and Acaryochloris thomasi (RCC1774). This suggests that these cyanobacteria may use a similar strategy to adapt to their light environments.
Each PSII-Pcb monomer contains four Pcb proteins acting as the light-harvesting system, which increases the overall antenna cross-section to enhance the light-harvesting capacity of PSII. The well-defined cryo-EM map allowed the team to identify the isoform types of all Pcb subunits in the whole structure, which shows the binding of both PcbA- and PcbC-type Pcb antennas. Despite the PcbC, it may form a complex with PSI under iron stress conditions. Intriguingly, the IsiA protein, which is supposed to be induced under iron limitation and form a complex with PSI, is not observed in the PSII-Pcb structure. All these Pcb subunits contain six transmembrane helices, and their N-terminal and C-terminal regions are located on the stromal side. This is different from the typical structures of LHC protein found in green algae and higher plants, which have three transmembrane helices.
Detailed comparison shows some obvious differences between the PcbA-type and PcbC-type antennas in both the structure and pigment arrangement. PcbA antennas have a similar structure and pigment arrangement as the IsiA, whereas the PcbC antenna exhibits some different structures and binding sites of pigment (Fig 3). In addition, the A. marina Pcb antennas contain a number of Zea similar to that of the Lhcr antennas in red algae PSI but different from the LHC antennas from other organisms, suggesting that the Pcb antennas may play an important role in photoprotection in A. marina.
Overall, the existence of different types of Pcb proteins and their specific structural features suggest that Pcb antennas are important and essential for the supramolecular organization of PSII and photoacclimation of A. marina to shade habitats and far red light-enriched conditions.
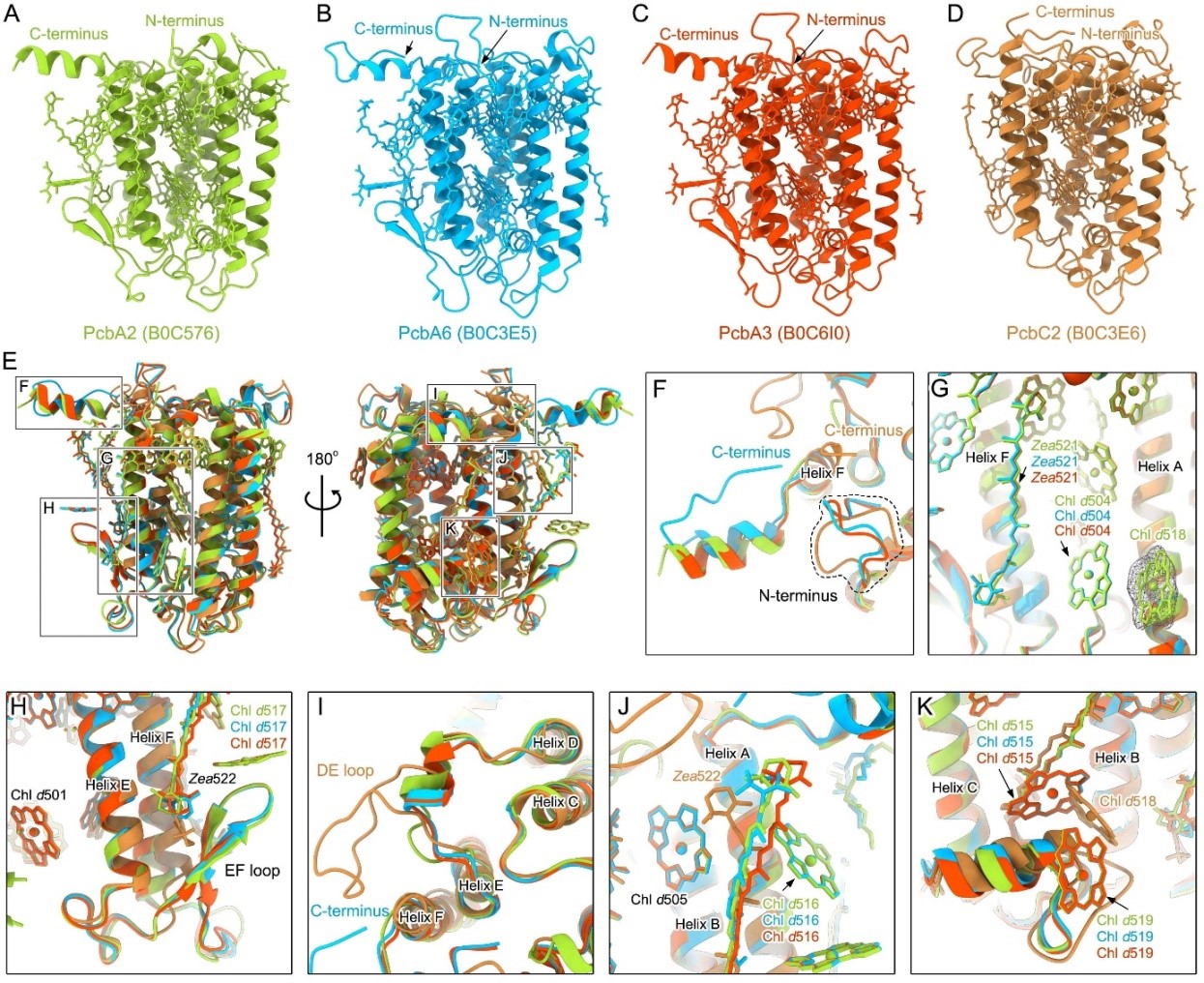
Figure 3. Structures of four Pcb antennas and their comparisons
Because A. marina has a unique Pcb arrangement unlike green algae and higher plants, the assembly between Pcb antennas and PSII core are different from the structures of PSII-LHCII seen in green algae and higher plants. In the PSII-Pcb structure, all the Pcb antennas exist as monomers, so the monomeric Pcb antennas are directly bound to the PSII core through the intrinsic light-harvesting systems CP43 and CP47 (Fig 4).
A previously unidentified Chl molecule was found in each of the CP43 and CP47 subunits, which likely facilitates the connection between the PSII core and Pcb antennas and enables the efficient energy transfer between the Pcb and PSII core. An unidentified protein was found at the antenna interface of the PSII-Pcb dimer, which acts like a hook to connect the Pcb antennas of two PSII monomers and ensures the stability of the dimer within a PSII-Pcb tetramer. Furthermore, strong interactions are found between the PSII core and Pcb antenna subunits of two PSII dimers, supporting the binding of two PSII dimers in the PSII-Pcb tetramer structure.

Figure 4. Interactions among different subunits of the PSII-Pcb tetramer of A. marina
The results challenge the existing understanding of the photosystem II structure and the mechanism of light energy utilization. They offer a structural foundation for revealing the molecular mechanism of far-red light utilization by Chl d-type cyanobacteria, supplying crucial insights into the diversity of light energy utilization and adaptation mechanisms among photosynthetic organisms. Furthermore, these findings serve as valuable guidance for the design of novel photosynthetic systems capable of utilizing a wide spectrum of light energy and for the development of high-light-efficiency crops.
SUSTech serves as the first and correspondent author for this paper. Liangliang Shen, a postdoctoral fellow, Dr. Yuanzhu Gao, an engineer at the Cryo-EM Center, and Kailu Tang, a doctoral student at Zhejiang University, are listed as co-first authors. Additionally, Professor Guangye Han from IB-CAS, Professor Peiyi Wang from SUSTech, and Professor Xing Zhang from Zhejiang University are co-corresponding authors. Academician Tingyun Kuang and Professor Wenda Wang from IB-CAS, along with Professor Min Chen from the University of Sydney, Australia, also contributed to this research.
This work was supported by the National Key R&D Program, National Natural Science Foundation of China (NSFC), Chinese Academy of Sciences’ Project for Young Scientists in Basic Research, China Postdoctoral Science Foundation, and the President Funding for Excellent Post Doctors at SUSTech.
Sample preparation was supported by the Public Technical Service Center of IB-CAS. Data collection and processing were completed at the Cryo-EM Center for SUSTech. Technical support was also provided by the Cryo-Electron Microscope Center and Protein Platform of Zhejiang University Medical College.
Paper link: https://www.science.org/doi/10.1126/sciadv.adk7140
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo ByCryo-EM Center