Biomedical alloys are widely used in biomedical materials and therapeutic devices, particularly for the replacement of hard tissues, which require excellent mechanical properties and biocompatibility. The low Young’s modulus of bone-replacing implants is an important constraint because a significant stiffness mismatch with the host bone can induce bone resorption owing to stress shielding, which may result in implant loosening and periprosthetic fracture.
Additionally, the ductility of biomedical alloys is a very important indicator during their service in human body and production processes. Therefore, an ideal biomaterial alloy should exhibit both a low Young’s modulus and relatively high ductility. However, traditional alloys have a limited range of Young’s modulus (45-210 GPa), which is significantly higher than that of human bones (~30 GPa). The alloys with low Young’s modulus usually have limited ductility. Therefore, how to obtain alloys possessing both a Young’s modulus close to that of human bones and good plasticity has become a technical challenge in the development of biomaterial alloys.
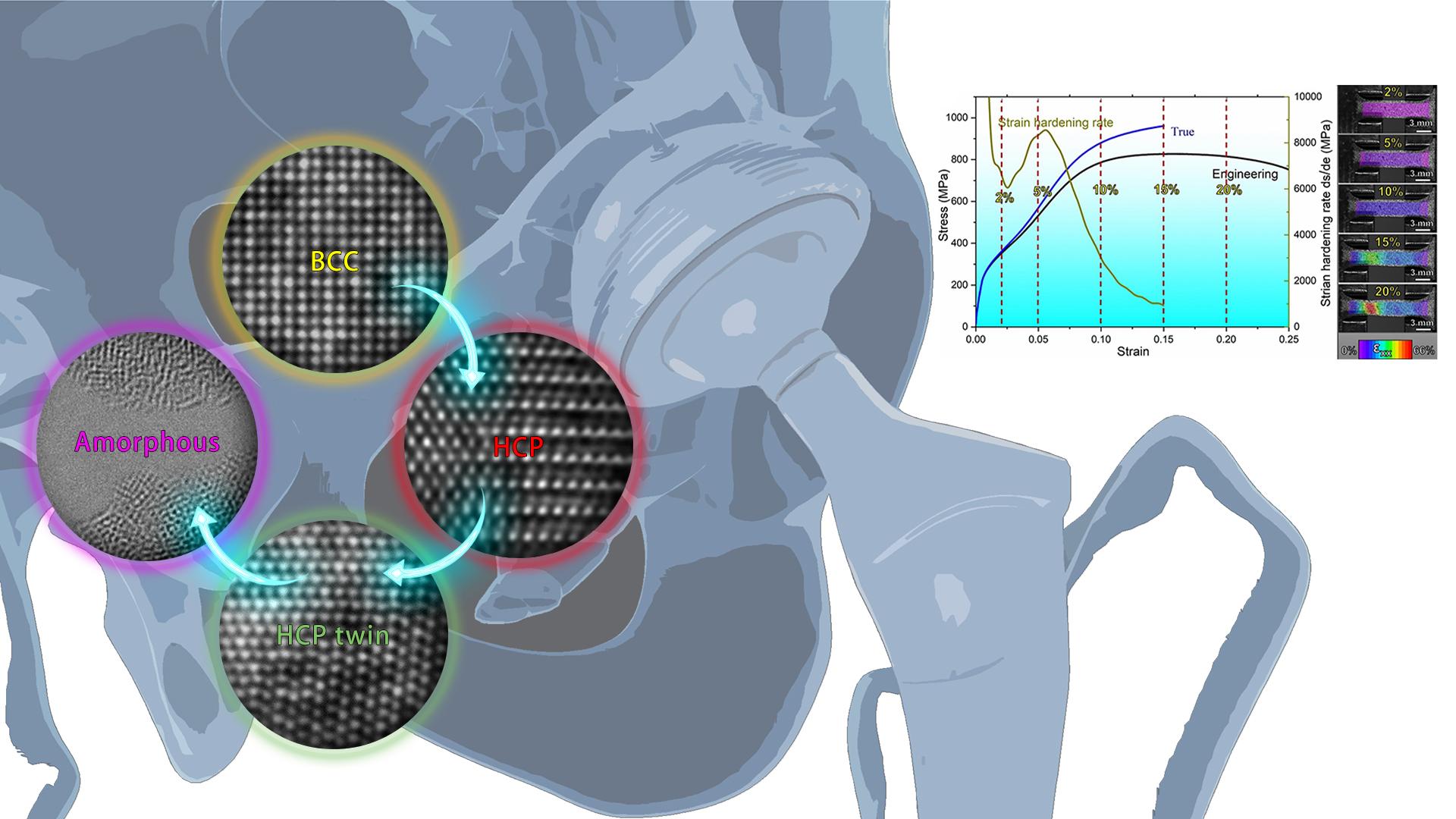
To overcome this aforementioned problem, Associate Professor Wenjun Lu’s research team from the Department of Mechanical and Energy Engineering (MEE) at the Southern University of Science and Technology (SUSTech), in collaboration with Dr. Xiaoqing Li from the Department of Materials Science and Engineering at KTH Royal Institute of Technology and others, have reported a metastable TiZrHfTa high-entropy alloy that exhibits an ultra-low Young’s modulus (29-37 GPa), excellent biocompatibility, and an acceptable ductility (~25%).
Their paper, entitled “An ultra-low modulus of ductile TiZrHfTa biomedical high-entropy alloys through deformation induced martensitic transformation/twinning/amorphization”, has been published in the high-impact journal Advanced Materials.
The researchers used first-principles calculations to select a single-phase metastable BCC (body-centered cubic) TiZrHfTa alloy composed of non-toxic elements. This alloy shows low stability and low bonding force, resulting in a low Young’s modulus. Additionally, this alloy exhibits good ductility, which was contributed by the introduction of stress-induced martensitic transformation, martensitic twinning, and amorphous transformation. Therefore, this metastable TiZrHfTa high-entropy alloy overcame the traditional bottleneck problem of biomedical alloys, which could not simultaneously achieve an ultra-low Young’s modulus and an acceptable ductility. As a result, this alloy has significant application prospects in biomedical applications.
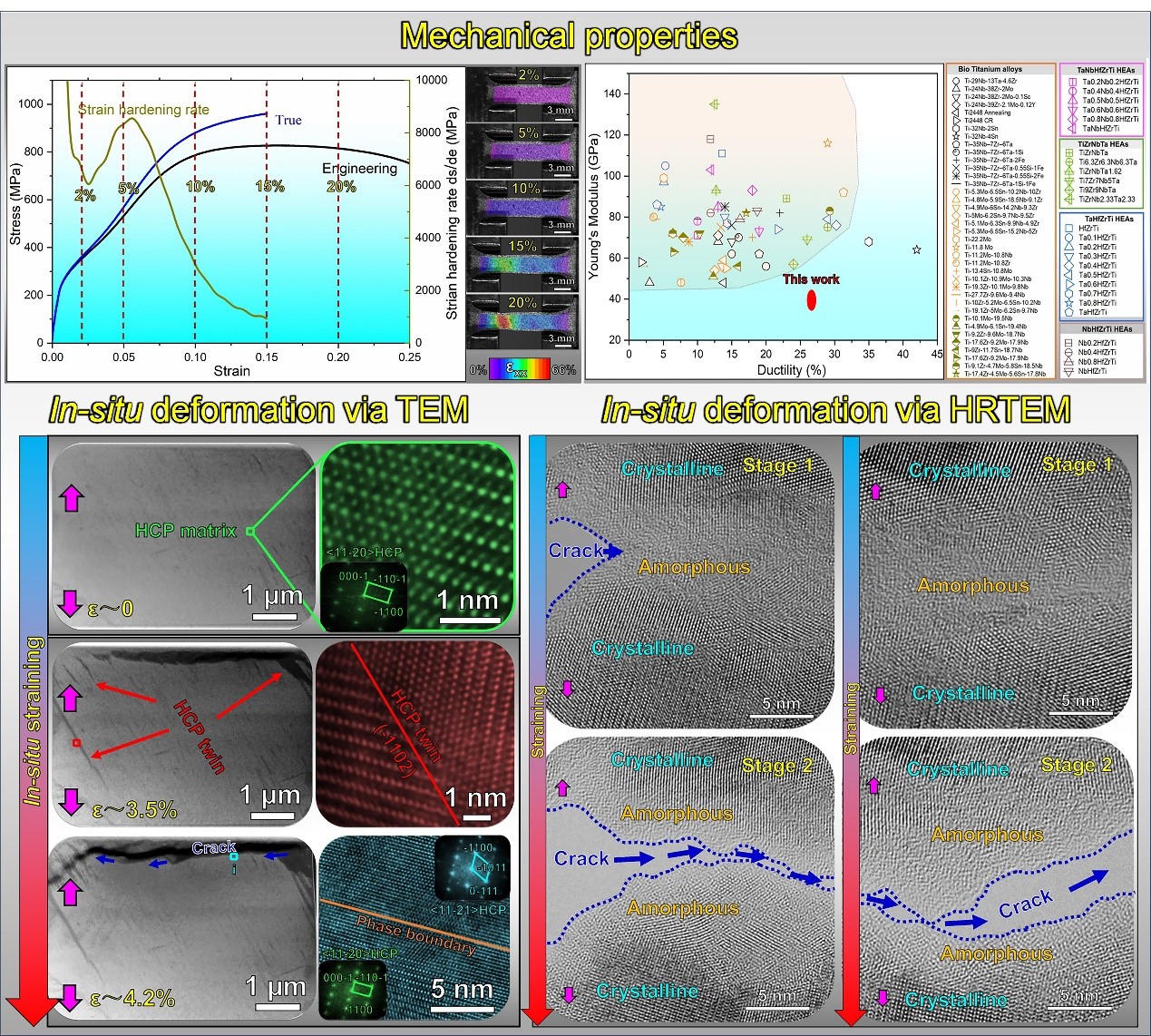
Figure 1. Mechanical properties of the TiZrHfTa Bio-HEA and the Young’s modulus and ductility profiles of the TiZrHfTa Bio-HEA and other biomedical alloys
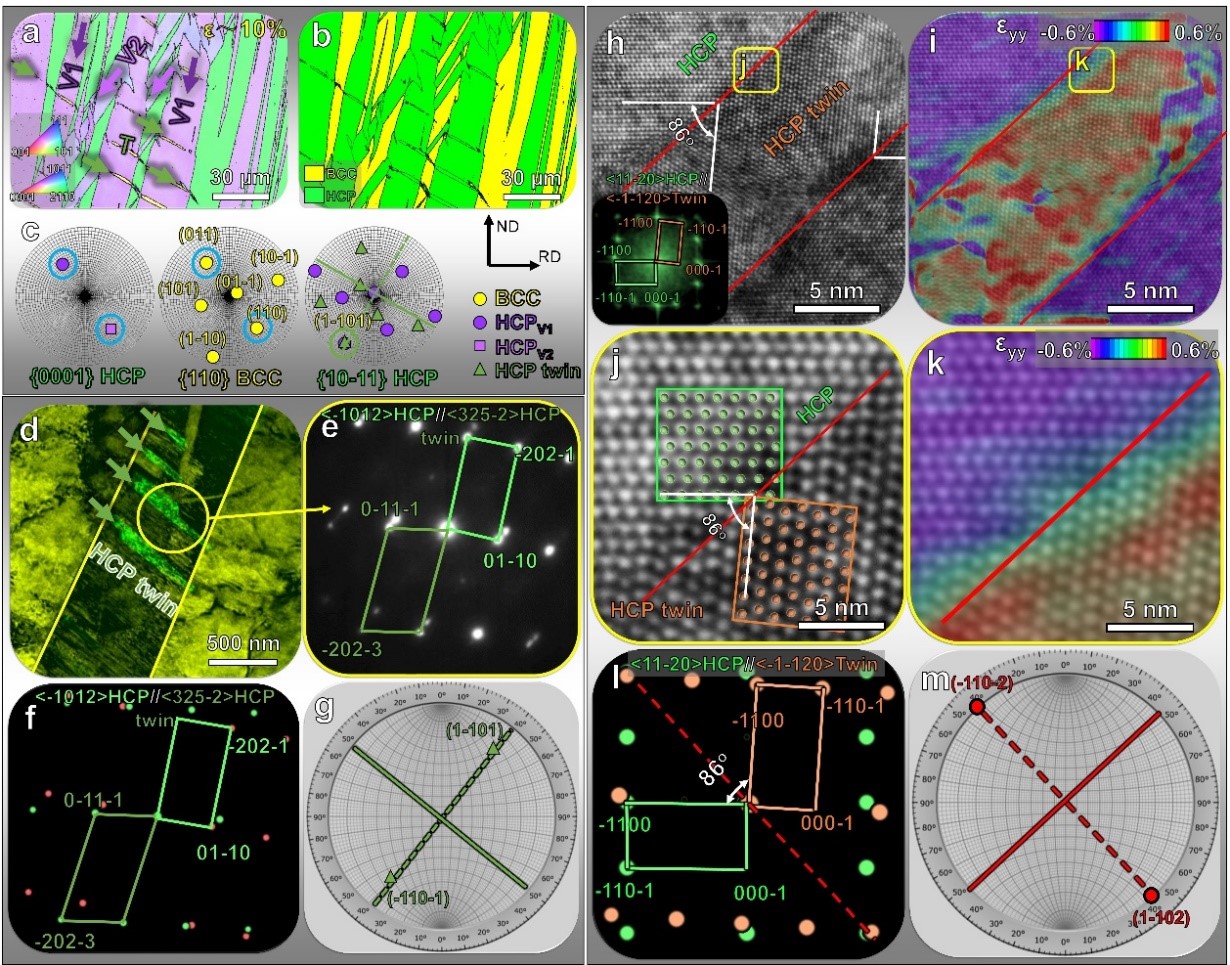
Figure 2. Multiscale characterizations of stress-induced martensitic transformation and twinning
Prof. Wenjun Lu’s team and his collaborators combined first-principles calculations with experimental methods to design and fabricate a kind of metastable BCC TiZrHfTa high-entropy alloy composed of non-toxic elements, exhibiting both low Young’s modulus and high ductility. They proposed a method to achieve a single phase with the lowest Young’s modulus among the constituent phases in the alloys by precisely tuning the stability of the body-centered cubic phase in Bio-HEA.
The subtle tuning of the body-centered cubic phase stability also enables the induction of stress-induced martensite transformation with extremely low trigger stress. The transformation-induced plasticity and work hardening capacity were achieved via the stress-induced martensite transformation. Additionally, the hierarchical stress-induced martensite twin structure and crystalline-to-amorphous phase transformation provided robust toughening mechanisms in the Bio-HEA. The cytotoxicity test confirmed that this Bio-HEA exhibited excellent biocompatibility without cytotoxicity.
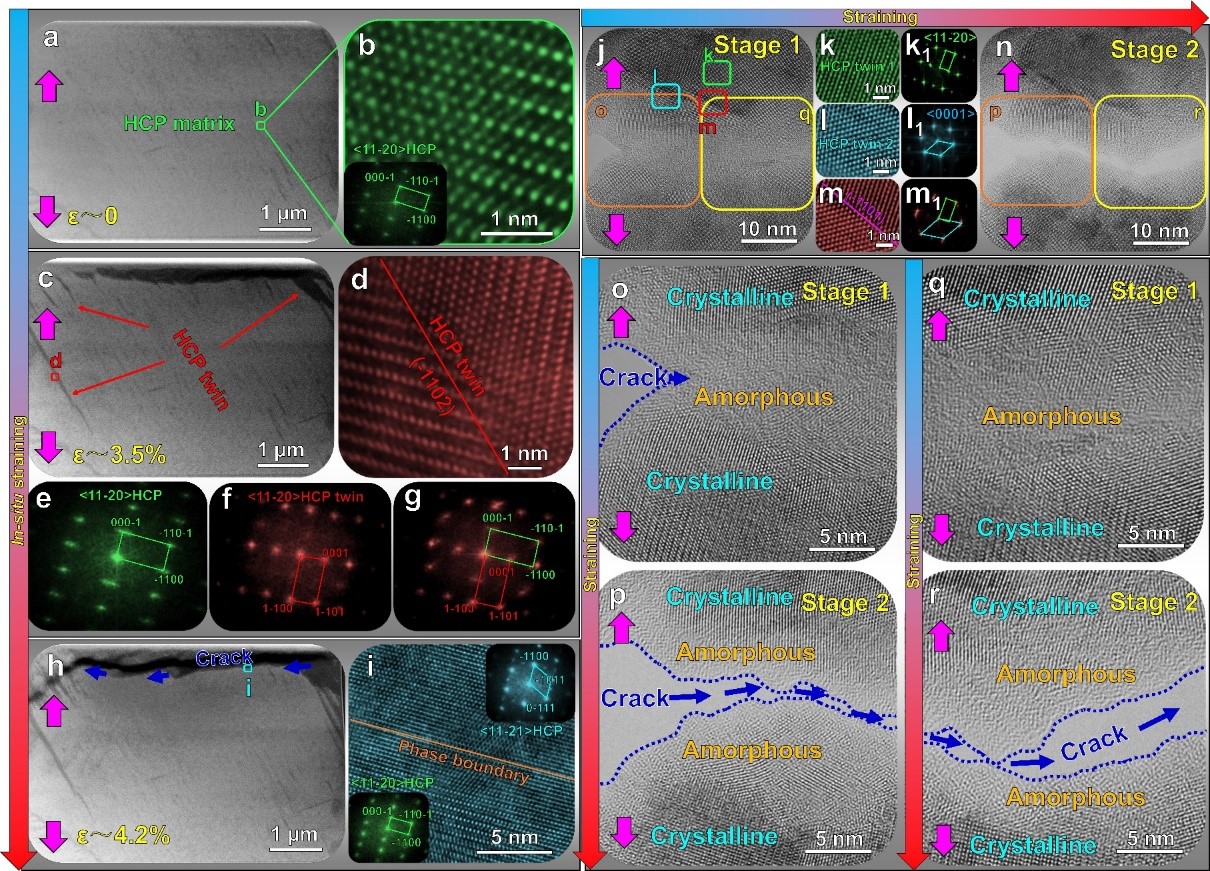
Figure 3. Nano-scale/atomic-scale in-situ tensile characterizations
Bingnan Qian, a postdoctoral fellow at SUSTech, is the first author of the paper. Associate Professor Wenjun Lu is the corresponding author, and the Department of MEE at SUSTech is the only corresponding institution. The first-principles calculations were conducted by Dr. Xiaoqing Li, while the biotoxicity testing experiments were completed by Dr. Yu Wang from Soochow University.
This work was supported by the Open Research Fund of Songshan Lake Materials Laboratory, National Natural Science Foundation of China (NSFC), Guangdong Basic and Applied Basic Research Foundation, Shenzhen Science and Technology Program, Swedish Research Council, Göran Gustafsson Foundation, Carl Tryggers Foundation, and the Swedish Foundation for Strategic Research.
The authors also acknowledge the Core Research Facilities of SUSTech and the National Academic Infrastructure for Supercomputing in Sweden (NAISS) at Linköping for providing technical support for this research.
Paper link: https://doi.org/10.1002/adma.202310926
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo ByDepartment of Mechanical and Energy Engineering