Hennipa virus belongs to the Paramyxoviridae family, whose main members include the Hendra virus (HeV) and Nipah virus (NiV), has the characteristics of extensive host distribution, cross-species transmission, human-to-human transmission, and high lethality. At present, the World Health Organization (WHO) has listed it as a key infectious disease for prevention and control, which belongs to the biosafety 4 (P-4) class pathogen. In 2022, a novel Hennepa virus, named Langya Hennepa virus (LayV), was discovered by screening fever patients in Eastern China. LayV may be transmitted to human populations either directly or through intermediate animal hosts and cause a range of respiratory symptoms in humans. Based on the serious harmfulness of HeV and NiV, high attention should be paid to this new Henipa virus, and its pathogenic mechanism should be deeply studied.
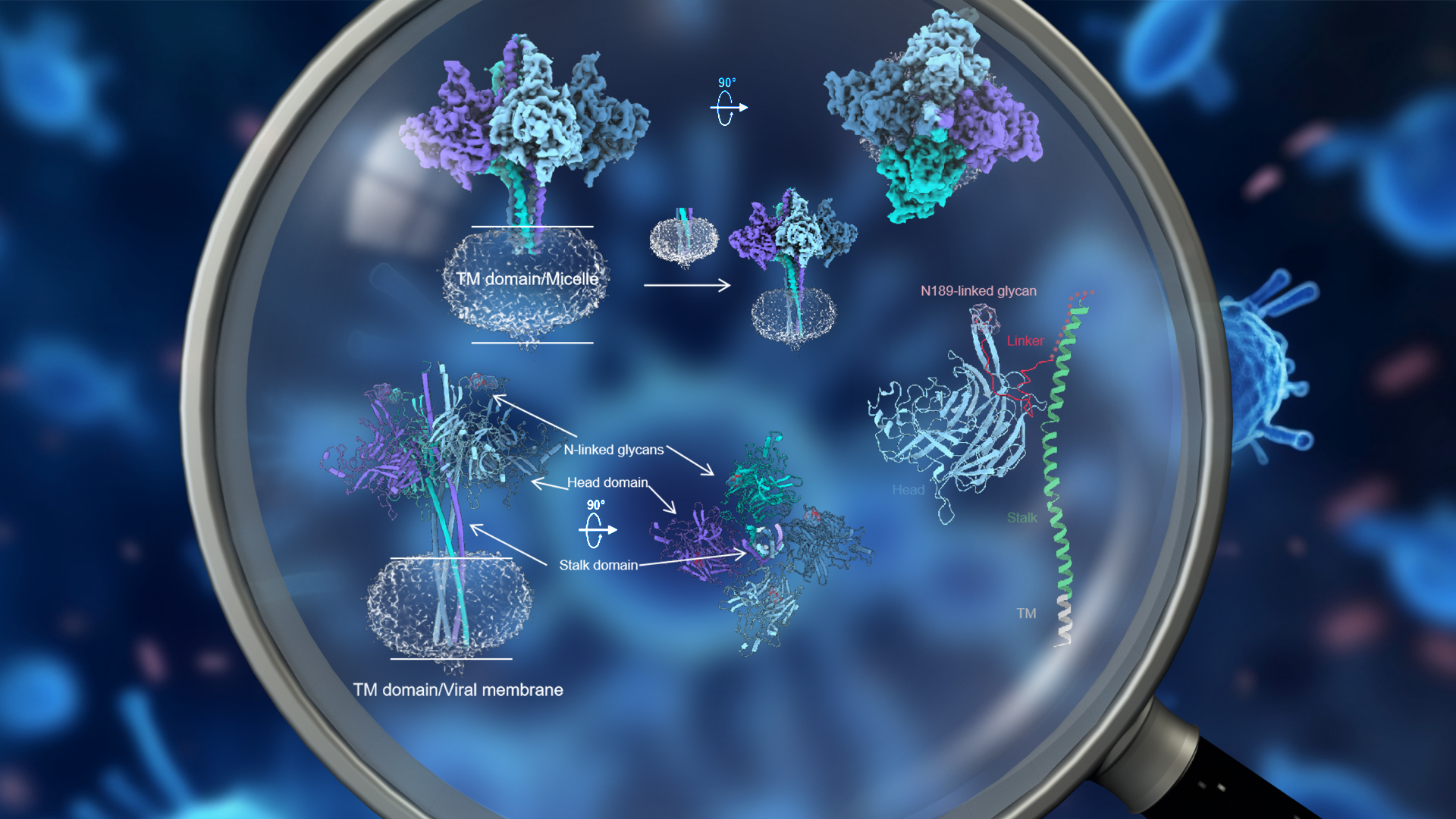
Assistant Professor Renhong Yan’s research team from the Department of Biochemistry at the Southern University of Science and Technology (SUSTech), alongside Professor Zheng Zhang and Researcher Bin Ju’s research team from the Institute for Hepatology of the Shenzhen National Clinical Research Center for Infectious Disease at the Shenzhen Third People’s Hospital (the Second Affiliated Hospital of SUSTech), have recently cooperated to publish their latest findings successfully resolving the near-full-length high-resolution structure of the key protein of Langya Hennipa virus (LayV) and the unique features of LayV-G protein in terms of structure, function, and immunology. Their work further increases the understanding of this novel Hennepa virus and provides an important reference value for the development of relevant vaccines, with great implications for the prevention and control of such new zoonotic infectious diseases.
Their work, entitled “The cryo-EM structure of homotetrameric attachment glycoprotein from langya henipavirus”, has been published in Nature Communications.
In this work, the researchers successfully established an in vitro expression and purification method of LayV-G protein, and resolved the nearly full-length cryoEM structure with 2.8 A resolution of LayV-G protein. Through comprehensive biochemical and cell biology studies, they found that LayV-G as a homotetrameric structure is different from the known resting state conformation of hnepal virus G protein tetramer, and also has large differences in its antigenicity.
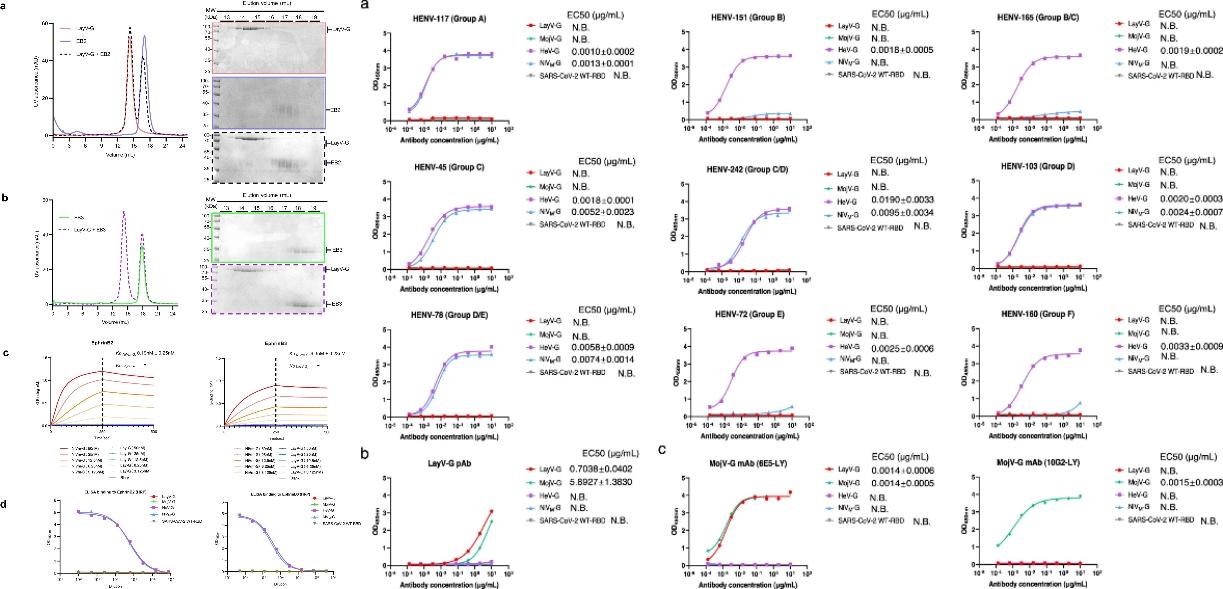
Figure 1. Biochemical characterization of the changes in the antigenicity of LayV-G
In order to explore whether the antigenicity of LayV-G changes, the researchers first used a variety of methods, such as in Vitro Incubation, Biological Layer Interference Technology (BLI), Enzyme-linked Immunosorbent Test (ELISA), and Flow Cytometer, to prove from biochemistry, biophysics, and cell level that LayV-G cannot be combined with known human Hennepa virus functional receptors ephrinB2 and ephrinB3.
Next, they evaluated the binding activities of NiV, HeV, LayV, and MojV G proteins using a series of monoclonal antibodies against six major epitopes of the Hendra virus G proteins. The results showed that the emerging LayV-G and the previously reported MojV-G have different antigenicity compared to the classical Hennipa virus G protein.
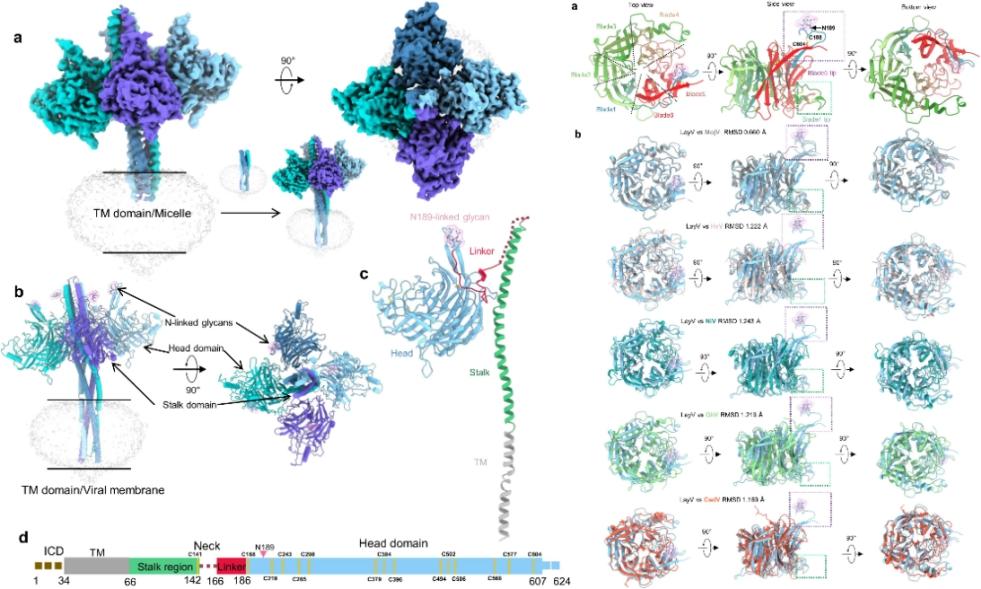
Figure 2. The overall structural diagram of LayV-G, head domain features, and structural alignment
To explain the above differences from the structural level, Professor Yan’s team and their collaborators successfully expressed and purified the full-length LayV-G protein sample, analyzing the high-resolution (2.8 A) structure of the extracellular region by cryoelectron microscopy for the first time, and locally optimizing the transmembrane region structure. A series of findings suggest that LayV-G and MojV-G may have similar novel mechanisms of host cell recognition. Thus, LayV-G is unable to interact with known Henipavirus receptors in vitro.
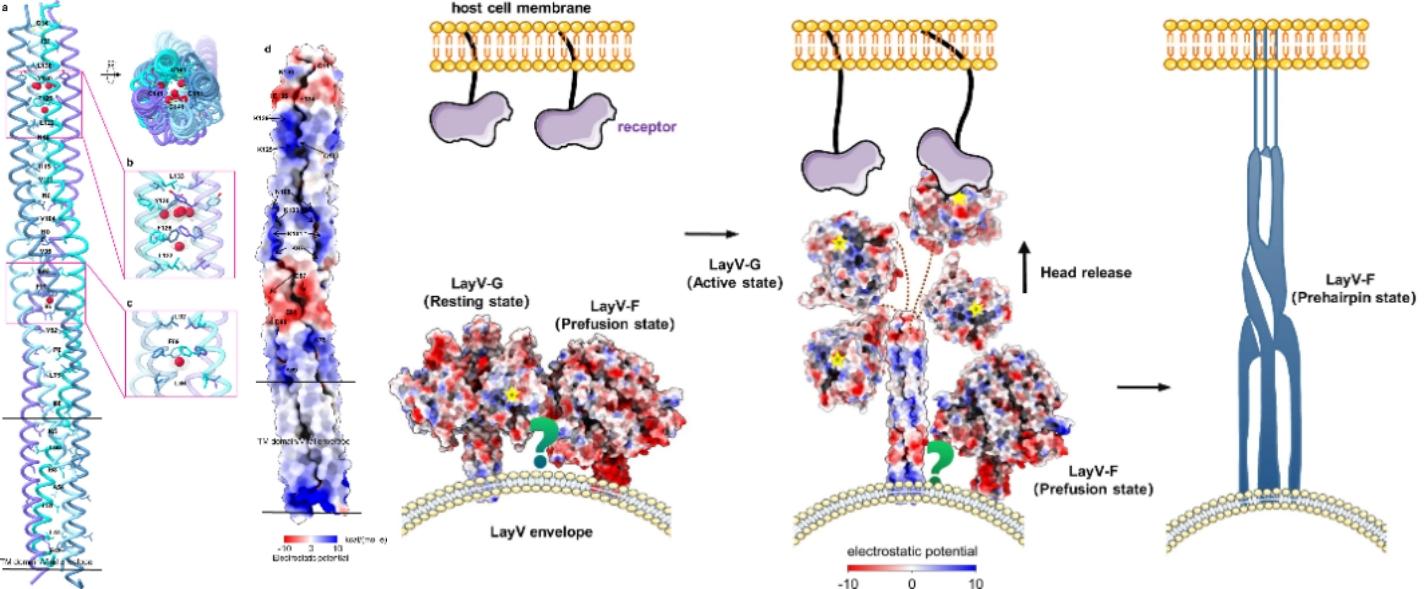
Figure 3. 4HB domain features and a diagram of the hypothesis model of LayV-G infecting host cells
In addition, they first observed the internal core region of the LayV-G 4 HB domain and found that the surface of the 4 HB domain and the transmembrane region showed a unique positive and negative potential distribution characteristics, which may be the key to the transition of LayV-G trigger LayV-F from the prefusion conformation, pre-hairpin conformation, and post-fusion conformation.
The tetrameric structure of LayV-G demonstrates the unique molecular structure of novel LayV, making it distinct from other Heniviruses. These findings not only enhance the understanding of the mechanism of emerging Hennepa virus entry into the cell, but also provide an important reference for the development of improved vaccine candidates with stability and immunogenicity.
Yingying Guo, a former Research Associate Professor from SUSTech (now a Researcher at the School of Life and Health at Hainan University), Songyue Wu and Wenting Li, a postgraduate student and postdoctoral fellow, respectively, from the Institute for Hepatology of the Shenzhen National Clinical Research Center for Infectious Disease at the Shenzhen Third People’s Hospital (the Second Affiliated Hospital of SUSTech), and Haonan Yang, a Ph.D. student from SUSTech, are the co-first authors of this paper. Assistant Professor Renhong Yan, Professor Zheng Zhang, Researcher Bin Ju, and Researcher Yingying Guo are the co-corresponding authors.
The study was supported by the National Natural Science Foundation of China (NSFC), National Science Fund for Distinguished Young Scholars, Institute of High-Resolution Biological Electron Microscopy, and the Science, Technology and Innovation Commission of Shenzhen Municipality. The researchers also acknowledge the Cryo-Electron Microscopy Center of SUSTech for providing technical support in electron microscopy data acquisition.
Paper Link: https://www.nature.com/articles/s41467-024-45202-5
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo ByDepartment of Biochemistry