Autophagy is a common protein degradation pathway found in all eukaryotic cells. During the formation of autophagosomes, plant cells retain many conserved components similar to other eukaryotic systems. Despite this similarity, it is still unclear how autophagosomes in plant cells acidify and mature, and how the mature autophagosomes are transported to vacuoles for degradation.
Studies in animal cells have shown that SNARE complexes, tethering proteins, and small GTPases play important roles in promoting the fusion of autophagosomes with the endo-lysosomal system and subsequent cargo degradation. However, no autophagosome-localized SNARE proteins have been identified in plant cells. In plants, only the HOPS complex exists among tethering proteins, and it is unclear whether it is involved in the regulation of autophagy.
In animal cells, RAB7 GTPase is a key upstream regulatory factor that recruits tethering proteins, thereby promoting the fusion of autophagosomes with the endo-lysosomal system. Whether RAB7 plays a role in the autophagy pathway of plant cells remains unclear. Additionally, vesicle transport mediated by ARF-like GTPase ARL8b and RAB7 plays an important role in the fusion of autophagosomes in animal cells. Currently, there are no reports of ARL8b homologous proteins in plants being involved in the regulation of autophagy. Therefore, how autophagosomes in plant cells integrate into the endo-vacuolar system for cargo degradation is almost completely unknown.
Associate Professor Ruixi Li’s research team from the Department of Biology, School of Life Sciences at the Southern University of Science and Technology (SUSTech) has uncovered a new mechanism by which the conversion from membraneless condensates to ring-like membrane-bound structures regulates autophagy degradation in plant cells.
Their work, entitled “A condensates-to-VPS41-associated phagic vacuoles conversion pathway controls autophagy degradation in plants”, has been published in the journal Developmental Cell.
In previous research, the team unexpectedly discovered that the evolutionarily conserved HOPS subunit VPS41 localizes at membraneless condensates associated with the endoplasmic reticulum. These condensates can exist independently of the HOPS complex and are not controlled by the upstream regulator RAB7 GTPase. The researchers focused on uncovering the cytological function of the membraneless condensates where VPS41 resides. They found that under various autophagy-induction conditions, the VPS41 condensates quickly lose liquid-like properties and transform into puncta and ring-like structures that encapsulate the ATG8 protein. Notably, these ring-like structures, which are acidic and do not colocalize with any known subcellular organelles, can exist independently of the HOPS complex.
Real-time observations showed that VPS41 initially interacts with the ATG8 protein in a punctate structure, gradually transforming into a ring-like structure that encapsulates ATG8. The process of VPS41 conversion from condensates to ring-like structures coincides with autophagy progression (Figure 1A). Immunoelectron microscopy results indicated that this ring-like structure is a double-membrane organelle, with VPS41 protein enriched in the lumen between the two membranes, while ATG8 is distributed both between the two membranes and within the inner membrane (Figure 1B). Given its unique ultrastructural characteristics and lack of significant colocalization with other organelles, the researchers named this structure the VPS41-associated phagic vacuole (VAPV).
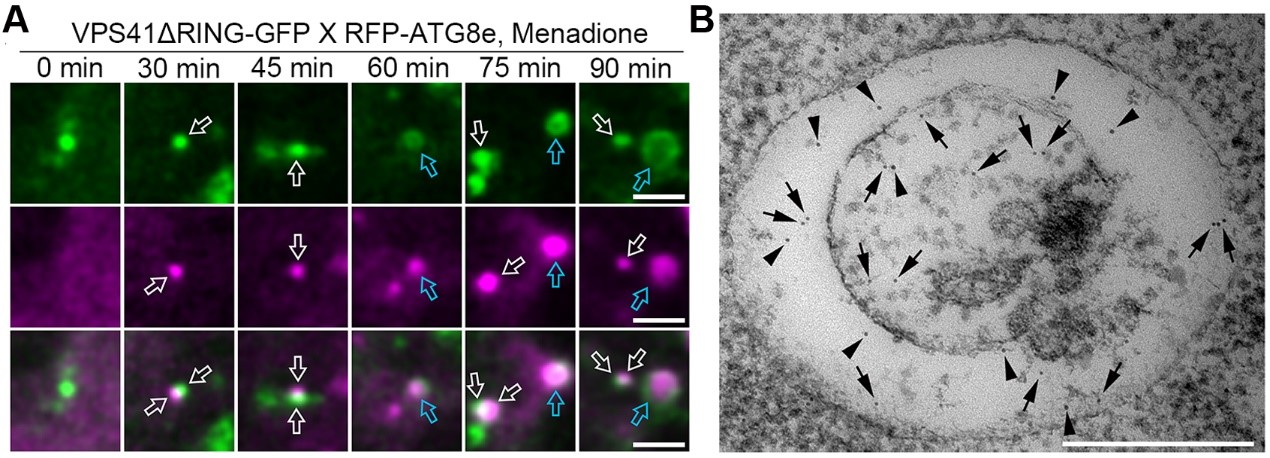
Figure 1. VPS41 is converted from condensates to VAPVs after autophagy induction
The team then explored the role of VAPV in the autophagy pathway of plant cells. They first discovered that mutations in VPS41 severely disrupt autophagy degradation. Further studies revealed that both the VPS41 condensates and their conversion into VAPVs under autophagy-induction conditions are not regulated by RAB7, and that the RAB7 pathway does not significantly affect autophagy degradation in plant cells. This represents a significant difference from other eukaryotic systems.
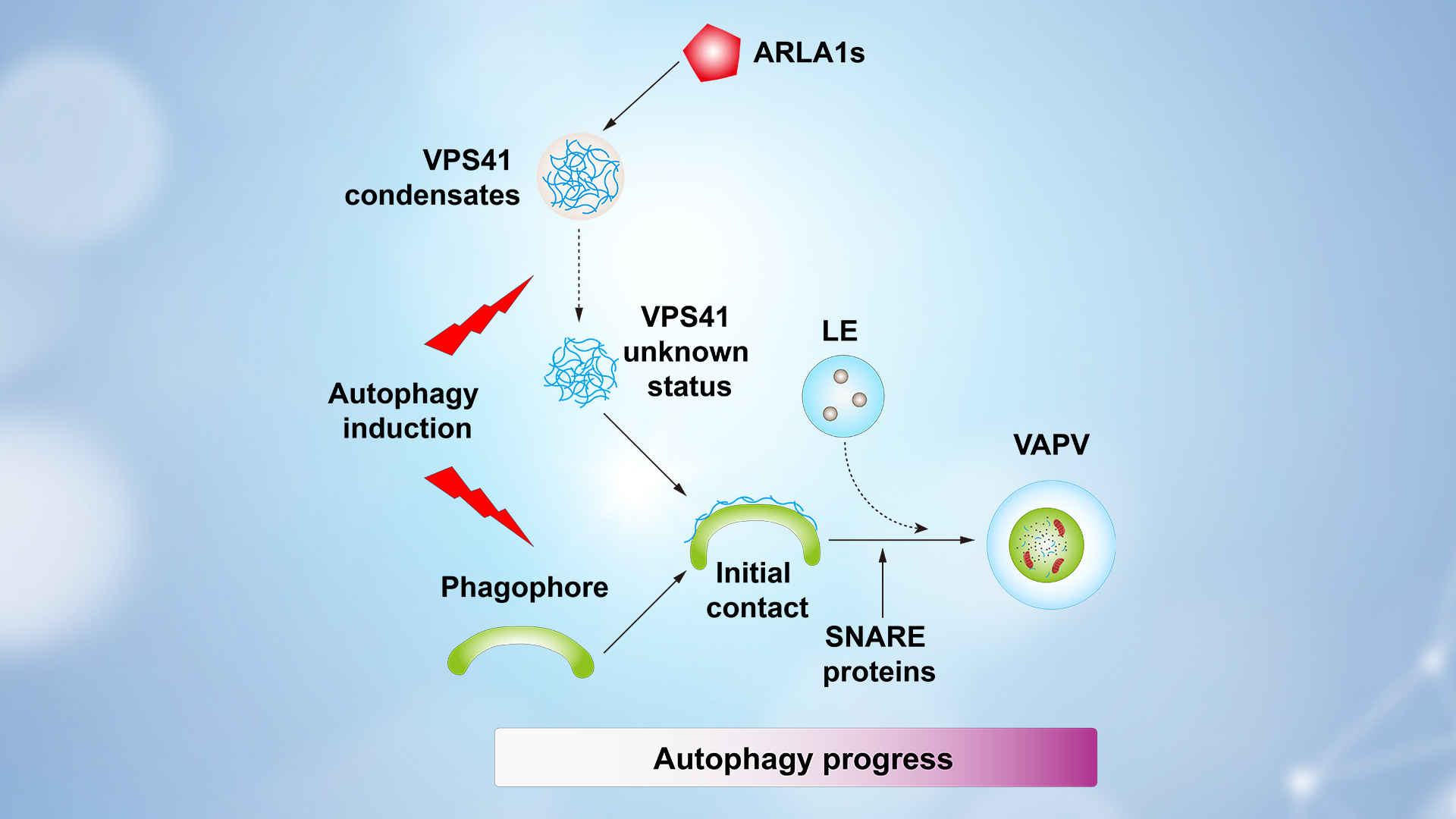
Further investigation found that ARLA1s, homologous proteins of ARL8b, as upstream regulators that promote the formation of VPS41 condensates, while SNARE proteins are key regulators to facilitate the conversion from VPS41 puncta to VAPVs. When this process is disrupted, autophagosomes can form but cannot be transported to vacuoles for degradation, leading to the accumulation of PE-conjugated ATG8 and a significant reduction in the cleavage of GFP-ATG8 under autophagy-induction conditions. The corresponding mutants exhibit hypersensitivity to carbon and nitrogen starvation. Therefore, the pathway by which VPS41 converts from condensates to VAPVs is crucial for autophagy degradation in plant cells.
The researchers also identified intermediate structures before the formation of VAPVs. In these structures, late endosomes (LEs) merge with the phagophore before closure, suggesting that VPS41 may promote the fusion of LEs with late-stage phagophores by recruiting SNARE proteins, ultimately forming VAPVs. Further exploration of the relationship between this pathway and the classic autophagy pathway revealed that the conversion from VPS41 punctate to VAPVs depends on the classic autophagy pathway. In ARLA1s mutants, autophagosomes can be produced, but most autophagosomes have abnormal structures with multilayers. Thus, the conversion from VPS41 condensates to VAPVs is likely an integrated part of the autophagy process unique to plants.
Based on the above data, the team draw the final working model. Under normal growth conditions, ARLA1s act as the upstream regulator to promote the formation of VPS41 condensates. After autophagy induction, VPS41 rapidly loses its condensate characteristics and transforms into punctate structures that are associated with the developing phagophore. Alongside autophagy progression, VPS41 gradually promotes the fusion of the developing phagophore with LEs to form the acidic organelle VAPV, a process dependent on the classical autophagy pathway and SNARE proteins (Figure 2). This work indicates that the development of phagophore and their fusion with acidic organelles to form mature autophagosomes is a two-in-one process unique to plants.
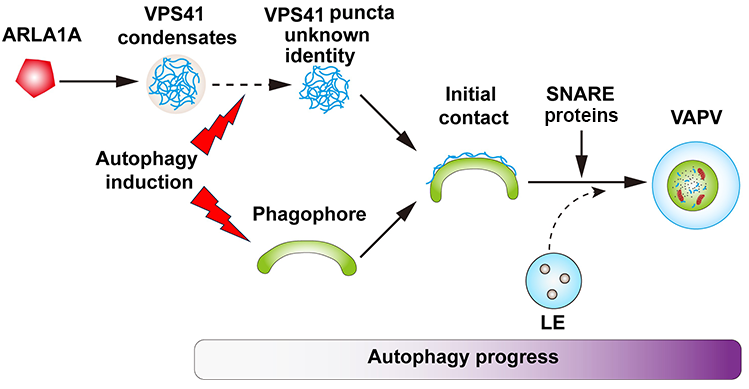
Figure 2. Working model
Doctoral student Dong Jiang and postdoctoral fellow Yilin He are the co-first authors of the paper. Associate Professor Ruixi Li is the corresponding author, and SUSTech is the first affiliated unit. Other contributors to this study included Professor Liwen Jiang from The Chinese University of Hong Kong and Professor Yi Zhang from Beijing Normal University.
This work was supported by the National Natural Science Foundation of China (NSFC), Shenzhen Stable Support Fund, Shenzhen Basic Scientific Research General Program, and the Shenzhen Key Laboratory Project.
Paper link: https://www.cell.com/developmental-cell/fulltext/S1534-5807(24)00447-7
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo ByDepartment of Biology