The interactions between proteins are essential for orchestrating the life processes within cells. It’s widely accepted that in a solution, a single protein can engage with multiple others, and in the case of competitive binding, it selectively attaches to one. But whether this is the only way occur inside cells?

A recent collaborative study by Associate Professor Cong Yu’s team from the Department of Chemical Biology and Associate Professor Zhiyi Wei’s group from the Department of Neurobiology, both of the School of Life Sciences, the Southern University of Science and Technology (SUSTech), have published a study that introduces a novel mechanism for how competitive binding influences interactions at the microscale level within protein condensates.
Their research, entitled “CLASP-mediated Competitive Binding in Protein Condensates Directs Microtubule Growth”, has been published in the prestigious journal Nature Communications.
The study delves into the role of the CLASP2 protein in relation to microtubules, which are fundamental to cellular structure, acting as conduits for molecular transport and facilitating organelle positioning and signal transduction. Microtubules are vital for maintaining cell shape, ensuring the proper distribution of chromosomes during cell division, and are guided to specific destinations through protein interactions at their ends.
CLASP proteins are pivotal in anchoring at the plus ends of microtubules, where the TOG4 domain of CLASP interacts with CLIP170 to stabilize its location. The TOG4 domain also interfaces with proteins such as LL5β at focal adhesions, GCC185 in the Golgi apparatus, and CENP-E at kinetochores, aiding in the precise placement of CLASP and microtubule ends. While previous studies have shown these interactions aid in microtubule targeting, the molecular underpinnings are not fully understood.
The researchers began by examining the structural biology of CLASP2’s TOG4 domain, which binds to a common motif, the TOG4 binding motif (TBM), present in various proteins that interact with CLASP. Cellular experiments confirmed that this interaction is crucial for CLASP2’s distribution in regions like microtubule plus ends and focal adhesions. Given the shared binding pattern, these TBMs can compete for binding to CLASP2. Further biochemical and cellular experiments confirmed this competitive binding.
The study also revealed that CLIP170 and the focal adhesion-associated protein ELKS1/LL5β undergo liquid-liquid phase separation, critical for the targeted localization of CLASP2 and microtubules. The competitive binding between CLASP2 and these proteins is instrumental in allowing the coexistence of multiple protein condensates without fusion, which is essential for microtubule positioning (Figure 1).
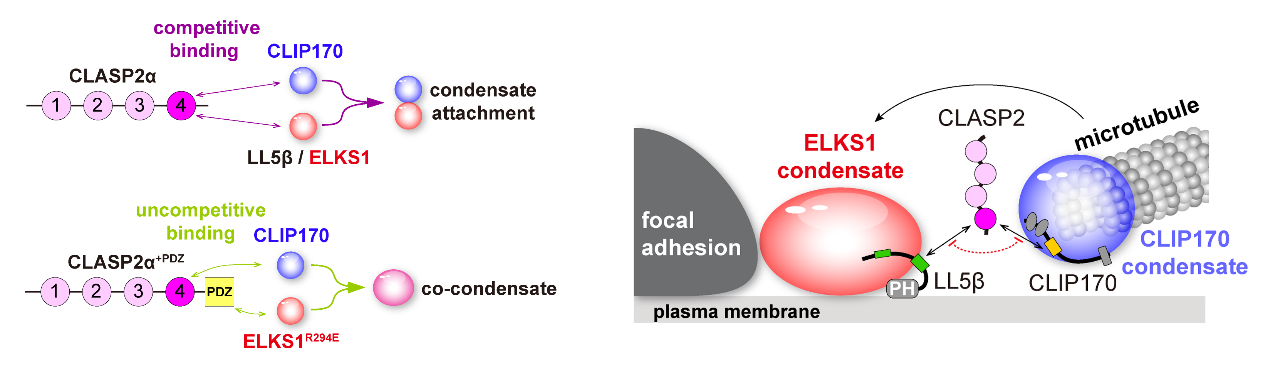
Figure 1. CLASP2-mediated competitive binding induces stable condensate interactions, thereby targeting microtubule plus ends to focal adhesions
Moreover, the study discovered that protein phosphorylation precisely regulates the interactions between condensates mediated by competitive binding. Other areas of the cell contain ELKS1 condensates that act as waypoints for microtubule growth (Figure 2). The kinase GSK3β modulates the interaction between CLIP170 and ELKS1 condensates through the phosphorylation of CLASP2, enabling dynamic contact and separation for directed microtubule growth.
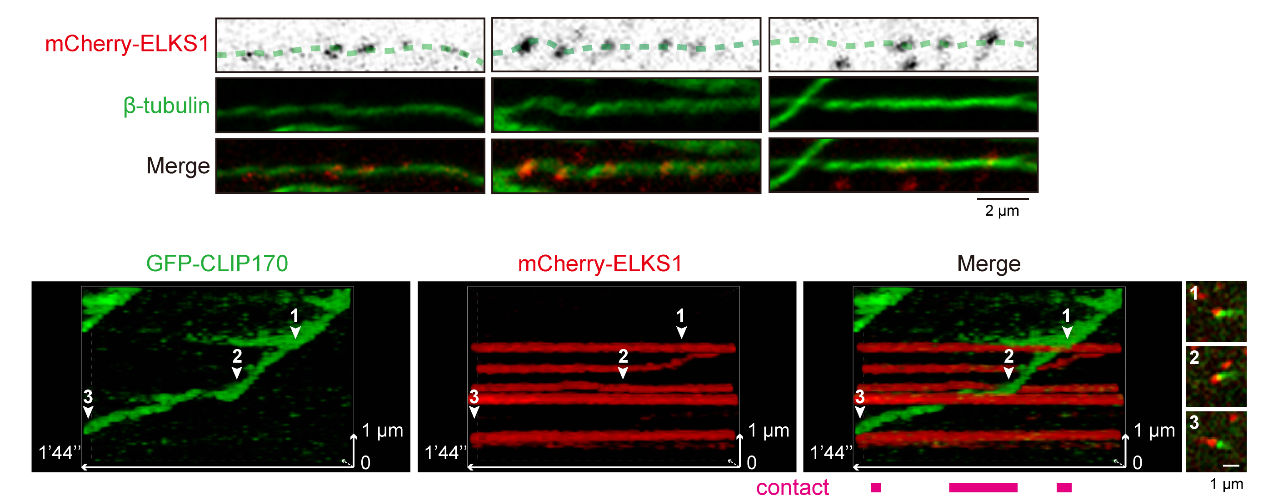
Figure 2. ELKS1 condensates can serve as “landmarks” that guide microtubule growth
Lastly, sequence analysis identified additional CLASP2-TOG4 interacting proteins with similar binding patterns. These proteins can also undergo phase separation and recruit CLASP2, further regulating microtubule interactions with cellular components (Figure 3). This discovery underscores a general mechanism for microtubule targeting and sheds new light on the intricate protein interactions at the mesoscale.

Figure 3. A schematic model illustrating how stable interactions between different condensates mediate microtubule targeting
Xuanyan Jia and Leishu Lin, Ph.D. candidates from the School of Life Sciences, are the co-first authors of the paper. Associate Professors Cong Yu and Zhiyi Wei are the corresponding authors. Professor He Sicong from the School of Life Sciences and Professor Yiming Li from the Department of Biomedical Engineering, along with their respective teams, made substantial contributions to this work.
This study was supported by the National Natural Science Foundation of China (NSFC), Guangdong Provincial Basic and Applied Basic Research Fund, Shenzhen Science and Technology Innovation Committee, Shenzhen-Hong Kong Institute of Brain Science, Shenzhen Key Laboratory of Biomolecular Assembly and Regulation, SUSTech Cryo-EM Center, and the SUSTech Core Research Facilities.
Paper link: https://www.nature.com/articles/s41467-024-50863-3
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo BySchool of Life Sciences