Mitochondria, often called the “powerhouses of the cell”, are responsible for producing ATP, the primary energy currency of cells. This process occurs in respiratory complexes located in the folded membrane structures called cristae within mitochondria. Typically, respiratory complexes I-IV form an electron transport chain to create a hydrogen ion concentration gradient, which is then used by respiratory complex V (ATP synthase) to produce ATP.
In brown adipocytes, this process is uniquely modified. Brown adipocytes contain high levels of a special protein called UCP1 (uncoupling protein 1), which converts the energy from the hydrogen ion concentration gradient into heat instead of ATP synthesis. This makes brown adipocytes that directly produce heat rather than storing energy as ATP.
Previous studies have shown that in cold-adapted organisms, the cristae structure in brown adipocyte mitochondria undergoes changes that lead to increased heat energy production. An encoded protein from the PERK gene was known to influence these cristae structural changes, along with increased activity of respiratory complex supercomplexes (RCS). However, the biochemical interaction linking PERK-induced cristae structural changes to increased respiratory complex activity and heat production was not fully understood.
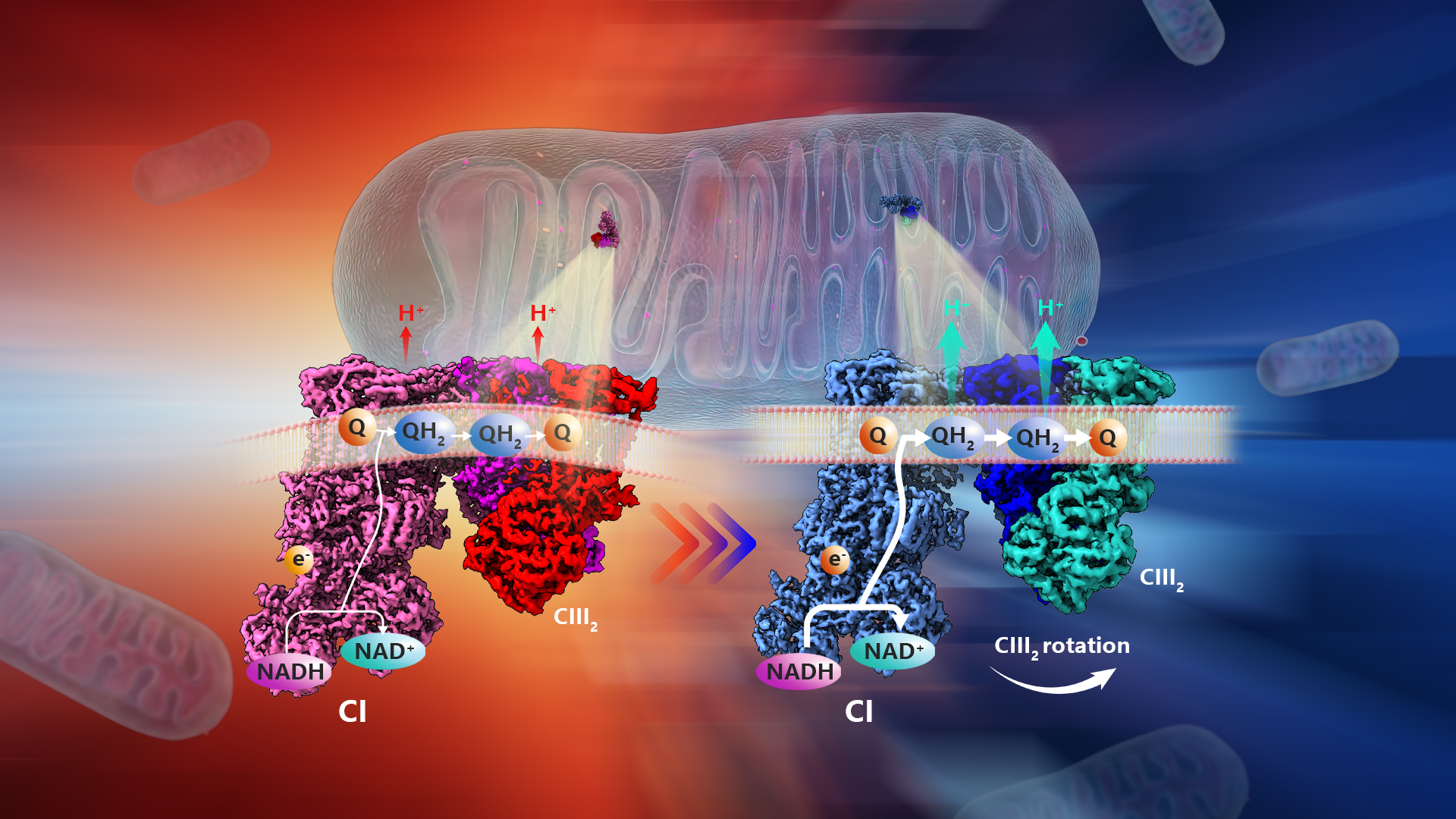
A team led by Chair Professor Maofu Liao from the School of Life Sciences at the Southern University of Science and Technology (SUSTech), in collaboration with Professor Pere Puigserver from the Dana-Farber Cancer Institute and Harvard Medical School, has now elucidated the mechanism underlying these structural changes in respiratory complexes of cold-adapted brown adipocyte mitochondria.
Their study, entitled “Structural basis of respiratory complexes adaptation to cold temperatures”, was published in the prestigious journal Cell on October 11, 2024.
The team conducted experiments by adapting mice to normal and cold temperatures, with the cold-adapted group further divided into wild-type and PERK mutant mice. The results showed that wild-type mice exposed to cold had increased cristae folds and enhanced respiratory complex activity in their brown adipocyte mitochondria. In contrast, mice grown at normal temperatures and cold-adapted PERK mutant mice did not show these changes, which is consistent with previous research.
Further analysis of the inner membrane of the brown adipocyte mitochondria from cold-adapted mice revealed a unique form of respiratory complex supercomplex (RCS). This new RCS had a distorted binding angle between complexes I and III, aligning with the altered curvature of the cristae. Computational simulations showed that this new structure could improve the efficiency of the electron transport chain.
This study demonstrates that PERK-induced cristae structural changes do not simply induce biochemical interactions or increase RCS expression, but instead induce the “behavior” of macromolecules through physical changes in membrane curvature. This provides the missing link between PERK, cristae structural changes, and increased RCS that was not previously elucidated. Moreover, this work is significant in analyzing various structural changes caused by intrinsic substrates at near-atomic resolution (about 3Å).
This discovery greatly enhances our understanding of cold adaptation mechanisms and is expected to provide new insights into energy metabolism and obesity-related research. Future applications based on these research findings are anticipated in various fields, including the treatment of metabolic diseases and the improvement of energy efficiency.

Figure 1. Structural adaptation of respiratory complexes from thermoneutral to cold temperatures
Associate Research Professor Young-Cheul Shin at SUSTech and Researcher Pedro Latorre-Muro at the Dana-Farber Cancer Institute are the co-first authors of this paper. Professor Pere Puigserver, Chair Professor Maofu Liao, and Researcher Pedro Latorre-Muro are the co-corresponding authors.
Paper link: https://www.cell.com/cell/abstract/S0092-8674(24)01087-0
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo BySchool of Life Sciences