Autophagy is a crucial lysosomal-mediated degradation pathway in cells. Autophagy promotes cancer cell survival by eliminating detrimental materials, maintaining mitochondrial function, and recycling nutrients, thereby promoting cancer cells against adverse conditions (e.g., nutrient deprivation, chemotherapy, etc.). Understanding how autophagy works at the molecular level can provides new insights and strategies for cancer therapy.
In mammalian cells, autophagy typically involves four stages: initiation, autophagosome maturation, autophagosome-lysosome fusion, and degradation. Multiple autophagy-related proteins (ATGs) were involved in autophagic process, in fusion step, Stx17 and Vamp8 of the SNARE complex (Stx17-SNAP29-Vamp8) facilitate the membrane fusion between autophagosome and lysosome to promote autophagy.
A research team led by Associate Professor Ying Sun from the Department of Systems Biology at the Southern University of Science and Technology (SUSTech) has discovered a novel mechanism that regulates autophagy in cancer cells. This research sheds light on the critical role of Migfilin in autophagy regulation, opening new avenues for targeted cancer therapies.
Their findings, entitled “Migfilin promotes autophagic flux through direct interaction with SNAP29 and Vamp8”, have been published in the Journal of Cell Biology.
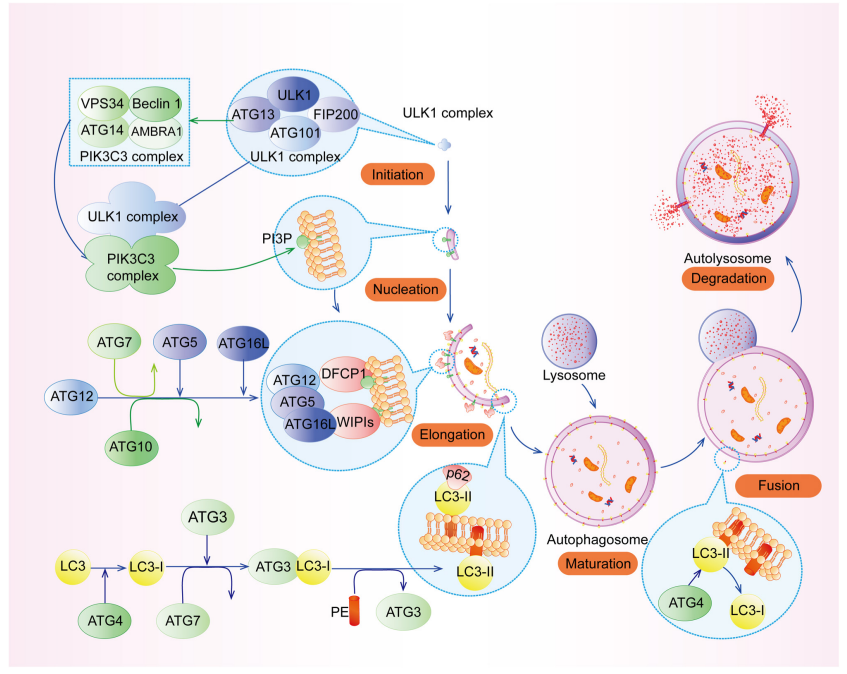
Figure 1. The autophagic process in mammalian cells (Xu, Yuan et al. 2020)
Ying Sun’s group found that Migfilin, a protein known for its role in focal adhesion, is also present in peri-nuclear regions in pancreatic cancer cells. They observed that Migfilin was localized at endosomes and lysosomes, suggesting its potential involvement in the autophagic process. The team found that Migfilin silencing led to a considerable increase of the p62/LC3 protein level and accumulation of autophagosome evaluated by GFP-LC3, indicating that normal process of autophagy was being blocked.
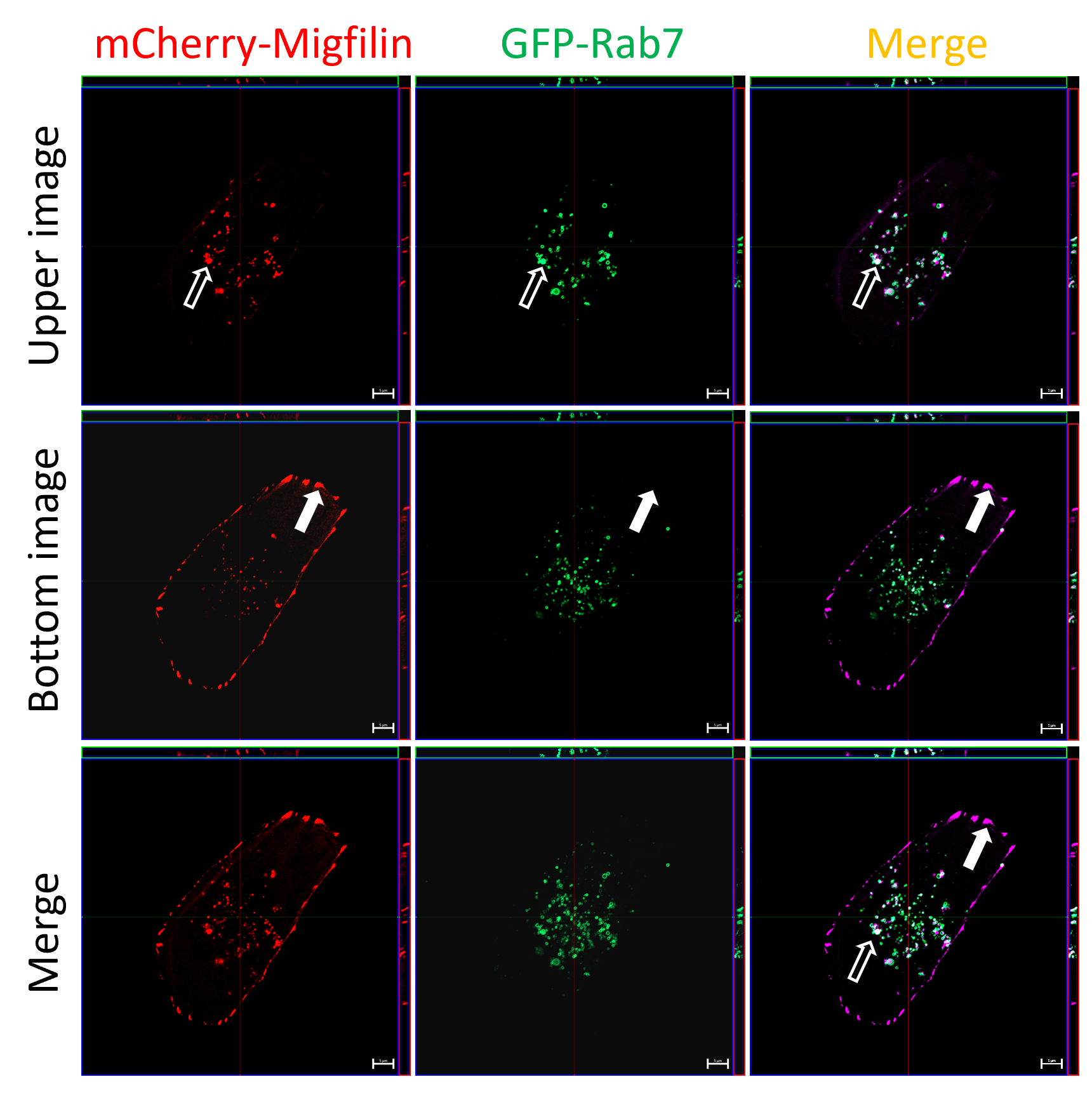
Figure 2. Migfilin localized at late endosome and lysosome
To explore the specific stage of autophagy where Migfilin plays a role, the research team investigated each step of process. Initially, they looked at autophagic initiation markers and found that Migfilin showed no significant effect on the initiation stage of autophagy. Next, they examined how Migfilin impacted the formation of autophagosomes, including ATG14-GFP, GFP-ATG16L, and mCherry-Stx17 puncta, and discovered that Migfilin silencing resulted in the accumulation of these early autophagic structures.
They found that Migfilin silencing inhibited the late stage of autophagy by employing the mRFP-GFP-LC3 assay to monitor the autophagic flux. Although it disrupted the process of autophagosome maturation, it did not affect the function of lysosomes. Finally, they confirmed that Migfilin played an important role in helping autophagosomes fuse with lysosomes, as shown by detailed studies of their interaction using co-localization analysis and TEM examination.
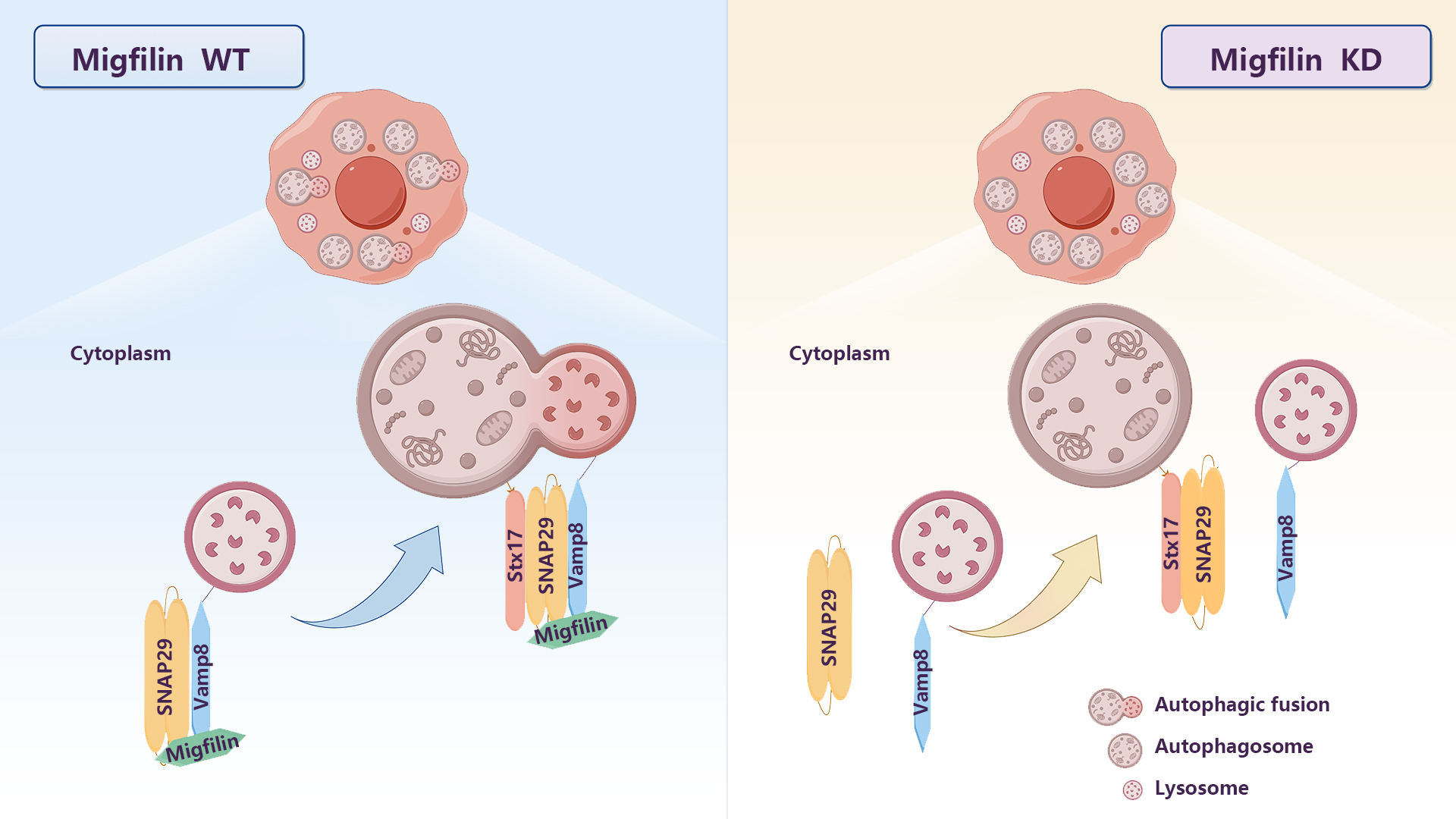
The researchers tried to understand how certain molecules work together in cells. They discovered that Migfilin binds to both SNAP29 and Vamp8, the components of the SNARE complex involved in autophagic fusion. They confirmed that both SNAP29 and Vamp8 directly interact with Migfilin through detailed experiments. Notably, Migfilin silencing inhibited the binding between SNAP29 and Vamp8, as well as cellular SNAP29-Vamp8 and SNAP29-lysosome/autolysosome interaction. This indicated that Migfilin functions as a scaffold protein to facilitate SNAP29 and Vamp8 bind and work properly.
To validate their observation, they enhanced the assembly of the SNARE complex through OGT silencing or using a mutant form of SNAP29 without O-GlcNAc modification. Both approaches successfully restored the impaired binding between SNAP29 and Vamp8, as well as the autophagic defects caused by Migfilin silencing. This supports the idea that Migfilin promotes autophagic fusion by improving the connection between SNAP29 and Vamp8.
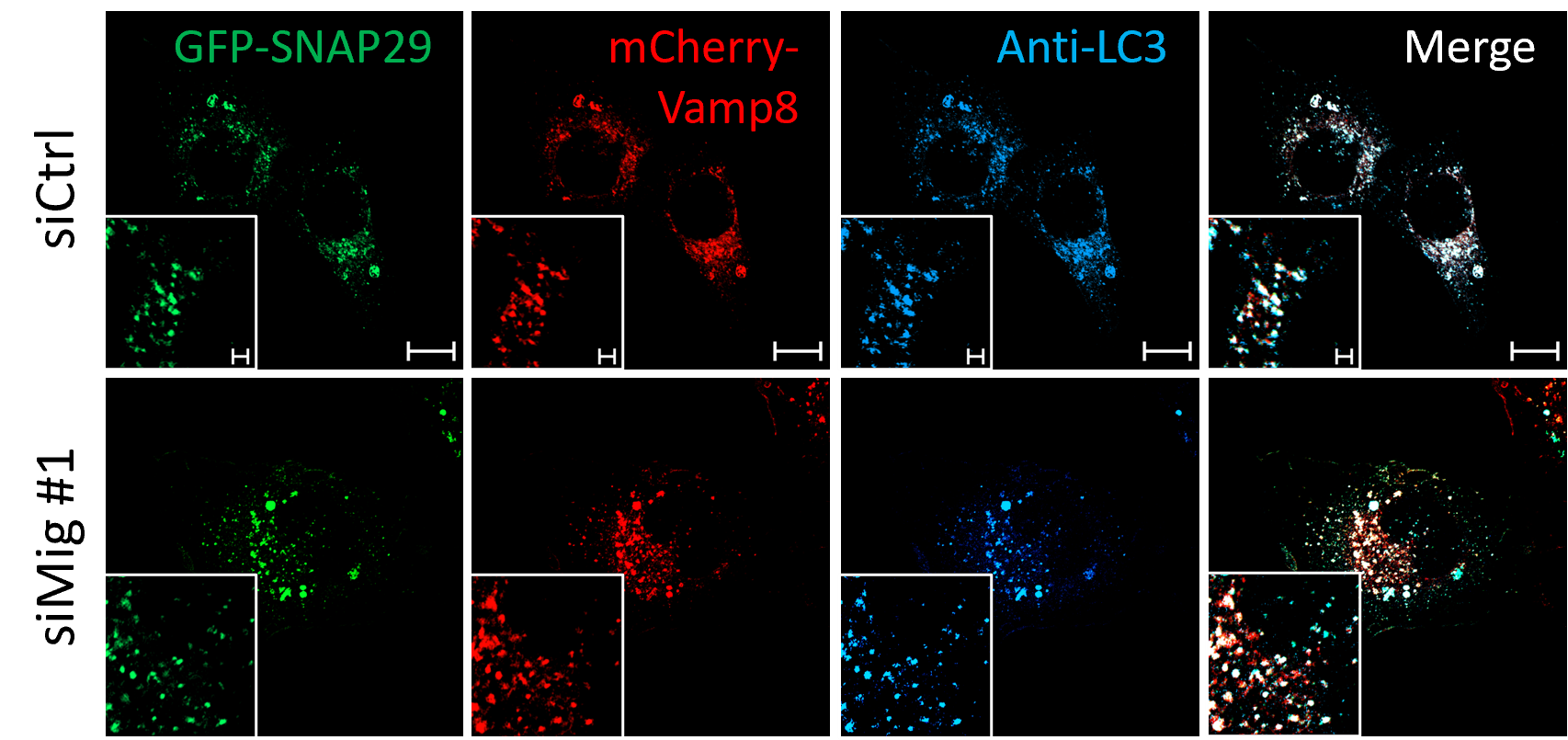
Figure 3. Migfilin deficiency inhibits intracellular SNAP29-autolysosome interaction
The team discovered that Migfilin plays a role in helping cells survive and maintain proper levels of focal adhesion protein homeostasis through the autophagy pathway. This study uncovers a new function of Migfilin as a regulator of autophagy and suggests that targeting the Migfilin-SNARE assembly could provide a promising therapeutic approach to help slow down cancer progression.
Ph.D. candidate Renwei Cai from the School of Life Sciences at SUSTech is the first author of the paper. Associate Professor Ying Sun and Professor Chuanyue Wu from the University of Pittsburgh are the co-corresponding authors, and SUSTech is the primary institution for this work.
Other contributors from SUSTech to this study include Associate Professor Yan Zhao, Zhiyi Wei, and Fuxing Zeng, Professors Ruijun Tian and Yi Deng, as well as engineers Wenjie Wei and Xibin Lu. Engineers Xixia Li and Xueke Tan from the Center for Biological Imaging of the Institute of Biophysics, Chinese Academy of Sciences, were also involved in this research.
Paper link: https://doi.org/10.1083/jcb.202312119
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo ByDepartment of Systems Biology