The direct air capture of carbon dioxide (CO2) with its subsequent conversion into renewable methane (CH4), the major component in natural gas, has garnered significant interest for several reasons. First, CH4 serves as an important chemical raw material for various chemical products (e.g., olefins and aromatics). Additionally, CH4 functions as a good hydrogen (H2) carrier due to matured storage and distribution network for natural gas. Furthermore, CH4 can be directly used as fuel for heating and power generation. With China’s long-distance natural gas pipeline network expanding to 124,000 kilometers by 2023, and over 4,000 kilometers added in the past year, the infrastructure for CH4 is well-developed.
Given the current immaturity of hydrogen technologies, it is more practical to produce renewable CH4 by reacting CO2 captured from the air with H2 generated through water electrolysis. This approach is particularly relevant in light of China’s “30-60” carbon peaking and neutrality goals, as well as the inevitable transition in energy structure. As a result, using renewable CH4 as an energy carrier for fuel storage aligns better with current need for sustainable energy solutions.
The research group led by Assistant Professor Meng Lin from the Department of Mechanical and Energy Engineering at the Southern University of Science and Technology (SUSTech) has achieved new progress in the field of direct air capture (DAC) and simultaneous CH4 synthesis. Their study presents a hybrid electro-thermochemical device capable of directly capturing CO2 from ambient air and converting it into CH4 in real-time. The innovative device features the cogeneration of CO2 and H2 in a single compartment using a bipolar membrane electrodialysis (BPMED) module, avoiding the need for a separate water electrolyzer. The process is followed by a thermochemical methanation reaction, resulting in CH4 production.
Their findings were recently published in Nature Communications under the title, “A hybrid electro-thermochemical device for methane production from the air”.
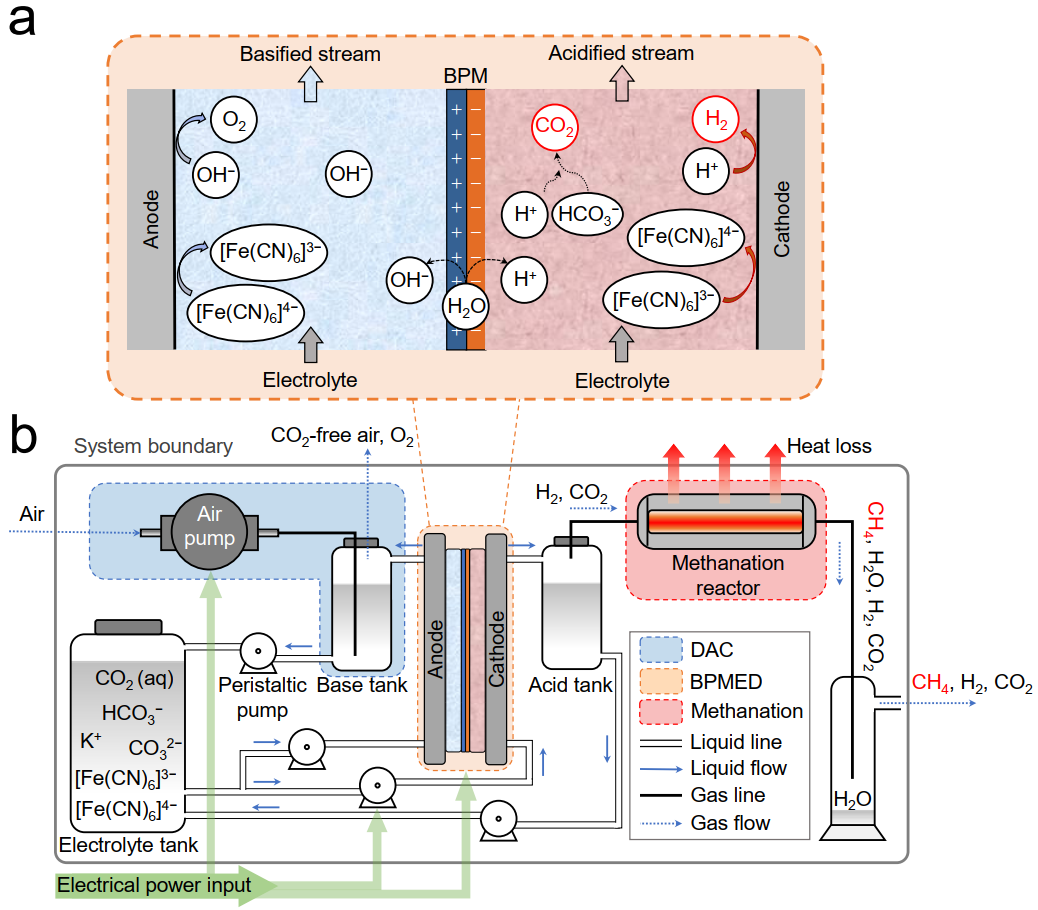
Figure 1. Schematic of the hybrid electro-thermochemical device for CH4 production from air
The hybrid electro-thermochemical device consists of three modules: a CO2 absorption module, a BPMED module, and a thermochemical methanation module. In the BPMED module, the water-splitting reaction at the electrodes is partially replaced with reversible redox couple reactions. This creates an acidic condition for CO2 release and hydrogen evolution reaction, as well as an alkaline condition for CO2 absorption and oxygen evolution reaction.
The device offers the following advantages. First, it reduces energy consumption reduction due to a simplified cell structure that requires fewer membranes and compartments, leading to lower resistivity and lower overall process energy consumption. Second, the simplified cell structure and the avoidance of vacuum-assisted gas stripping devices contribute to cost reductions. Lastly, the integrated approach enables simultaneous CO2 release and H2 production with tunable ratios (e.g., 1: 4 CO2 to H2), which is ideal for the subsequent methanation reaction (CO2 + 4H2 → CH4 + 2H2O). Overall, the device’s reaction can be summarized as CO2 + 2H2O → CH4 + 2O2.
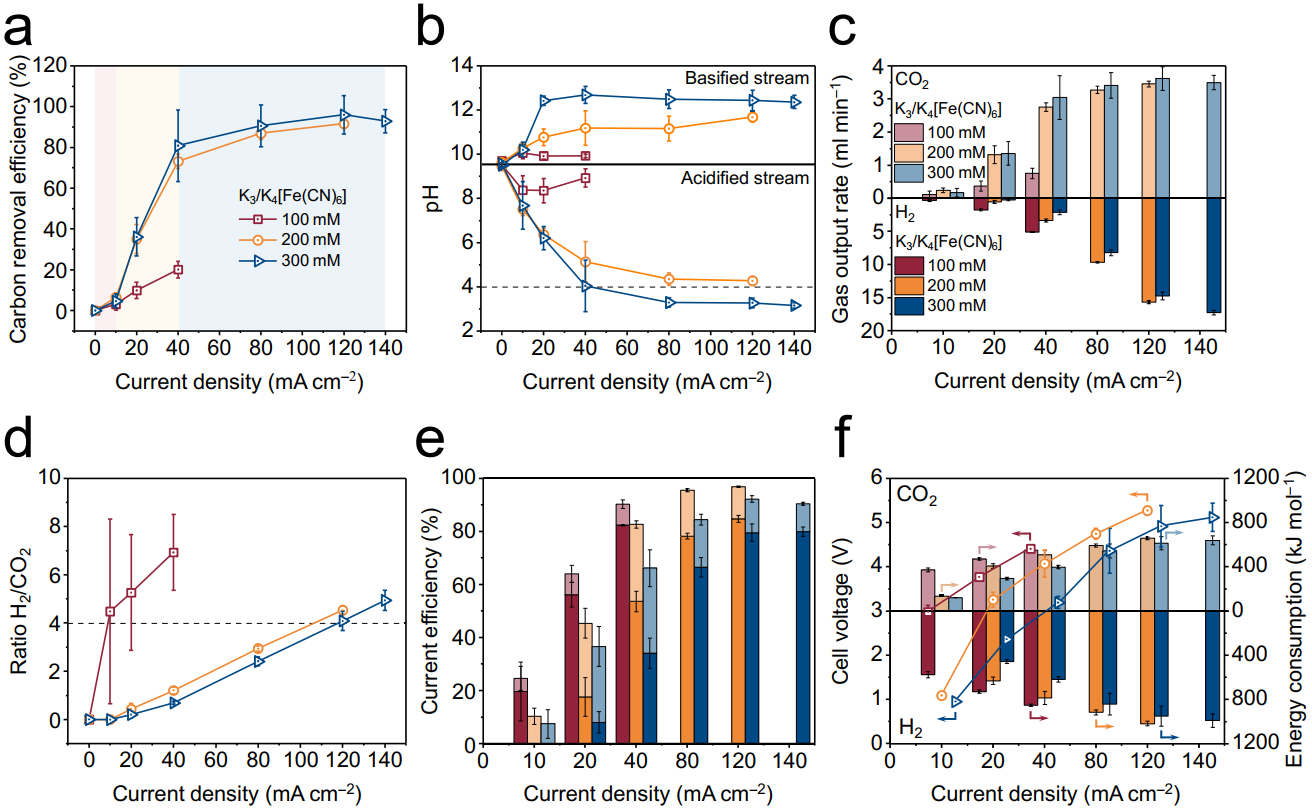
Figure 2. Performance tests of CO2 release and H2 production in the BPMED module
The BPMED module demonstrated high carbon removal efficiency and current efficiency. Moreover, the precise regulation of the H2-to-CO2 ratio can be achieved by controlling the current density, providing optimal conditions for subsequent methanation reaction.
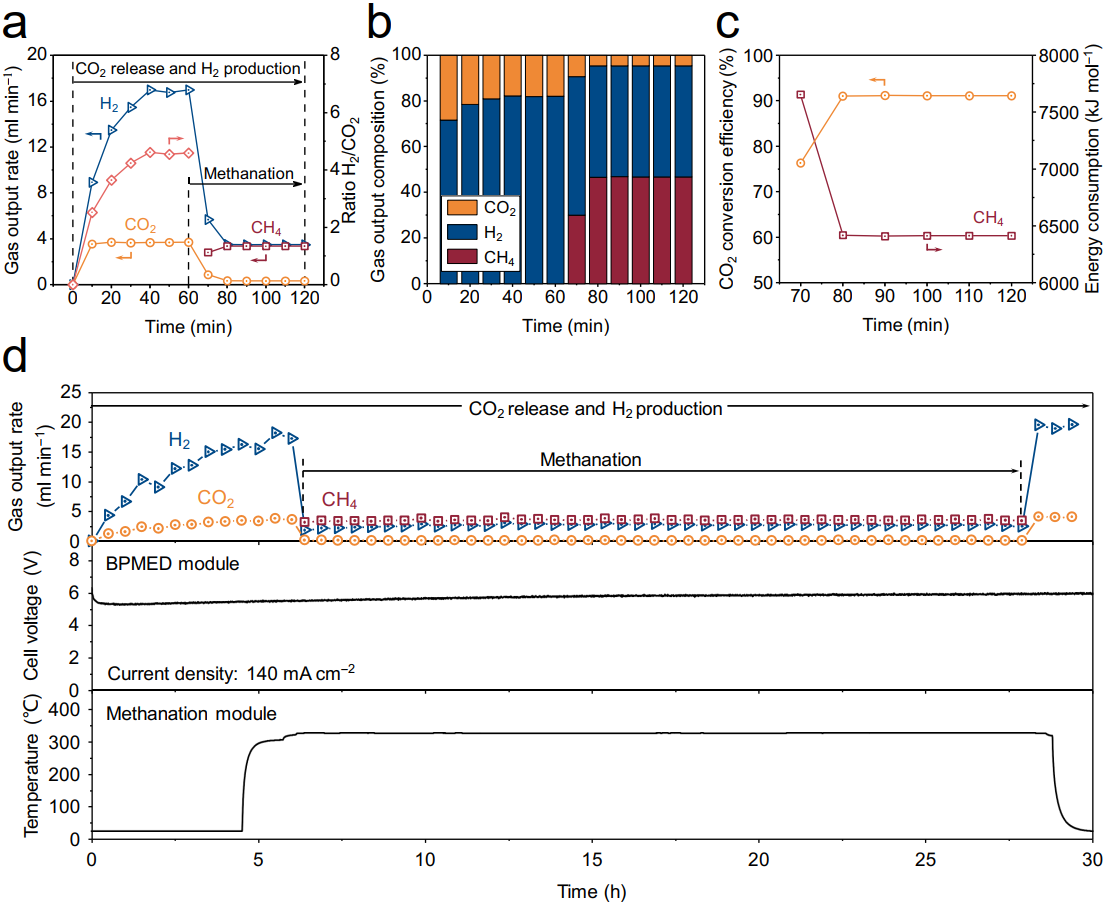
Figure 3. Simultaneous DAC and H2 production via a BPMED device cascaded with thermochemical methanation
Long-term system stability tests and real DAC experiments using indoor air as a diluted carbon source confirm the feasibility of capturing CO2 from air and converting it into CH4 in real-time. The proof-of-concept experiments show that the energy consumption for CO2 release and H2 production to be 704.0 kJ mol−1 and 967.4 kJ mol−1, respectively. The subsequent methanation reaction achieves a 97.3% CO2 conversion efficiency, with an electrochemical energy consumption of 5206.4 kJ mol⁻¹ for CH4 production.
By optimizing the BPMED module to reduce the overpotential, the energy consumption for CH4 production can be reduced by over 50%. Capturing CO2 from air and converting it into CH4 not only represents an innovative approach to addressing climate change but also offers an effective means of energy storage, economic development, and reducing dependence on fossil fuels.
Yaowei Huang, a visiting student from the Department of Mechanical and Energy Engineering, is the first author of the paper. Ph.D. student Da Xu, also from the same department, is the second author, with Professor Shuai Deng from Tianjin University and Assistant Professor Meng Lin serving as co-corresponding authors. SUSTech is the first affiliated institution of the paper.
Paper link: https://doi.org/10.1038/s41467-024-53336-9
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo ByDepartment of Mechanical and Energy Engineering