Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal cancers, with a five-year survival rate below 10% and a median survival time of less than six months. By 2024, PDAC has become the third leading cause of cancer-related mortality. The lack of reliable early diagnostic biomarkers and tenacious resistance to almost all existing therapies are major causes of poor prognosis.
Most cell-to-cell communication is initiated by secreted and plasma membrane (S‒PM) proteins, which are a rich source of biomarkers and therapeutic targets. PDAC’s tumour microenvironment (TME) is highly atypical and rich in stromal cells, which contributes to its dismal prognosis. However, proteome-level functional information on reciprocal signaling between stromal and cancer cells remains limited.
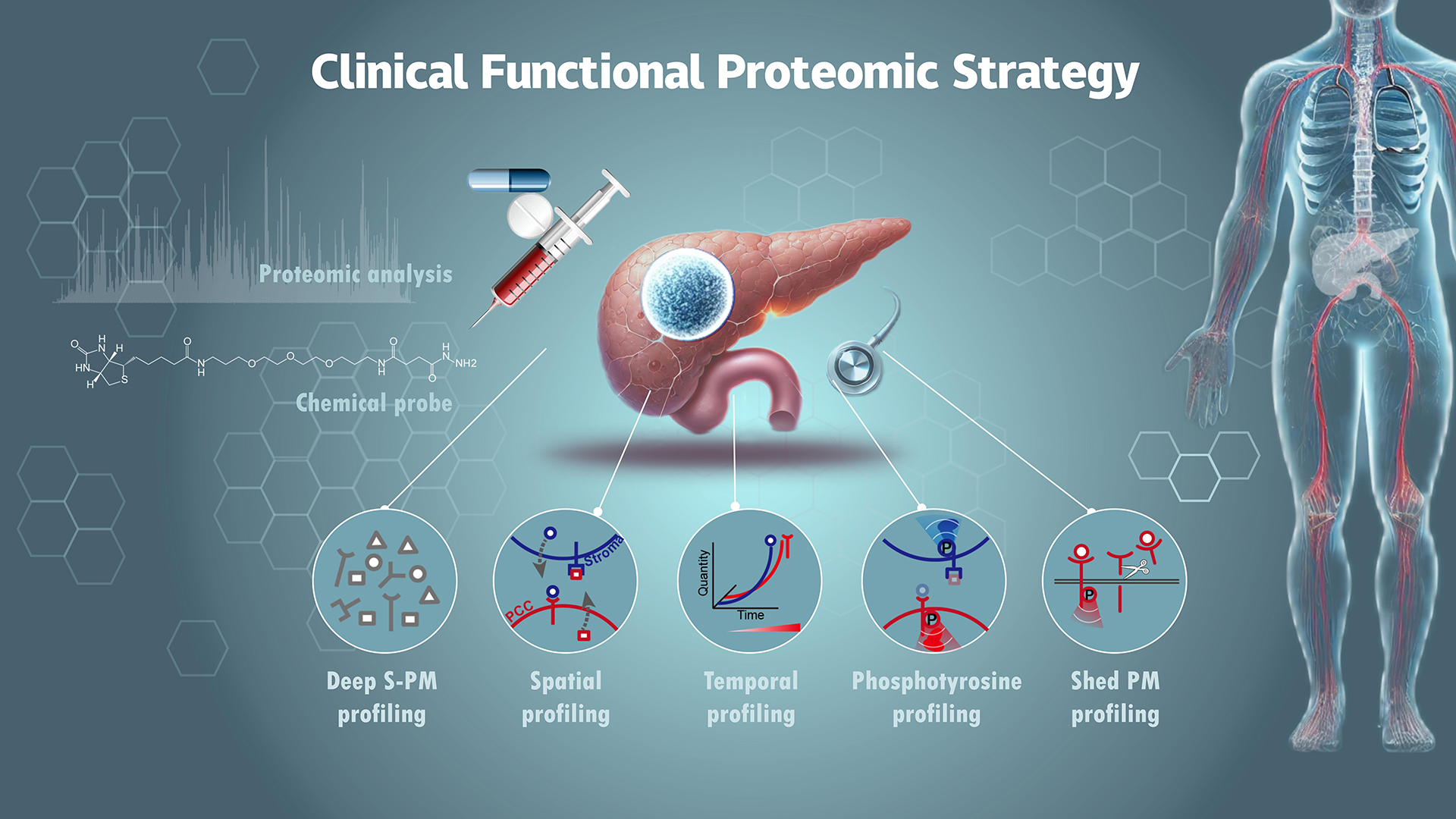
Professor Ruijun Tian’s research group from the Department of Chemistry at the Southern University of Science and Technology (SUSTech), in collaboration with domestic and international teams, has integrated multidimensional proteome data to reveal functional interplay between pancreatic cancer cells (PCCs) and stromal cells. This analysis, which includes both spatial and temporal resolution, was conducted on PDAC tissues from patients and a genetically engineered mouse model. This work provides a valuable resource for further functional and translational studies on PDAC.
Their paper, titled “Clinical functional proteomics of intercellular signaling in pancreatic cancer”, has been published in the journal Nature.
Unveiling the S-PM proteome of pancreatic tissues
The researchers addressed the heavily glycosylated nature of S-PM proteins by developing a glycan-based enrichment strategy. This enabled them to profile the S-PM proteome across 100 human pancreatic tissue samples (including tumours, paired non-cancerous adjacent tissues, chronic pancreatitis, and normal pancreatic tissues) to an unprecedented depth. Their findings included many well-characterized cancer biomarkers and protein targets with FDA-approved drugs, providing an untapped resource for investigating intercellular signaling in the PDAC TME.
To pinpoint the cell-type origin of the S-PM proteins and understand their potential intercellular signaling role, the team performed laser microdissection (LCM)-based spatial proteome and cell type-specific proteome profiling. This allowed them to map out cell type-specific proteomes for both PCCs and stromal cells in PDAC tumour tissues, producing the largest spatial proteome landscape of PDAC to date. Their dataset covered more than 76% of the S‒PM proteome identified in bulk tumour tissues and defined more than 200 paracrine signaling pairs between PCCs and stroma.
Using the genetically engineered KPC mouse model, they explored the S-PM proteome’s role during PDAC tumour progression. By collected tumour tissues from mice at various ages, (representing early, middle, and late tumour progression stages), they discovered that almost 90% of significant S‒PM proteins overlapped between humans and mice, exhibiting consistent expression trends in the two species. Additionally, they performed expression level-based clustering and found that these proteins were grouped into three clusters. Notably, one cluster showed upregulation from an early stage, containing various functional proteins likely critical for PDAC TME development and intercellular signal transduction.
Decoding intercellular signaling pathways in PDAC
Given the well-established role of phosphotyrosine (pTyr) machinery in activating early intercellular signaling, the researchers investigated key pTyr signaling in PDAC. They focused on identifying key components of the pTyr system, including pTyr writers (kinases), readers (proteins with SH2 or PTB domain), and erasers (phosphatases). These proteins frequently form complexes at pTyr sites, which indicate the activation of the signaling pathways. Their findings revealed a high coverage of these three protein classes, with more pTyr-activated proteins detected in tumour tissues compared to normal tissues.
Through systematic bioinformatic analyses, the team mapped the initial wave of intercellular signaling triggered by ligand-receptor interactions in PDAC tumours. They validated a novel intercellular signaling pathway in which PCC-derived PDGFs activate a PDGFR‒PTPN11‒ERK‒FOS axis to enhance LIF expression and secretion in PSCs (Figure 4h). In turn, PSC-derived LIF activates the LIFR‒GP130‒STAT3 axis in PCCs. This paracrine signaling pathway presents potential clinical applications by targeting these interactions to disrupt tumour growth.
In addition to tyrosine phosphorylation-mediated signal transduction, the researchers explored ectodomain shedding (ES) of plasma membrane (PM) proteins, which can modulate intercellular signaling by terminating receptor-mediated signals. Using a bioinformatic analysis pipeline, they identified 22 PM proteins that undergo ES in tumour tissues, with receptor tyrosine kinase AXL being the most significantly shed PM protein. Importantly, they discovered that matrix metalloprotease family members MMP1 and MMP11 act as new sheddases for AXL and other PM proteins, highlighting potential new targets for therapeutic intervention in PDAC.
AXL signaling as a therapeutic target
AXL, a member of the TAM family of RTKs, along with TYRO3 and MERTK, is aberrantly expressed in various cancer types and promotes chemoresistance and metastasis. In the current dataset, AXL was found to be more actively regulated than TYRO3 and MERTK, highlighting its important role in PDAC. To determine the extent of AXL shedding in tumours, for the first time, the researchers performed parallel reaction monitoring (PRM)-based targeted mass spectrometry (MS) analysis to measure in situ levels of full-length AXL (represented by the intracellular domain, or ICD) and its ectodomain (ECD), along with its ligand GAS6, in PCC and stroma regions across 50 tumour tissues. They found that approximately 70% of AXL was shed in both PCC and stromal regions.
When they compared tumour samples from patients with or without lymph node metastasis based on relative levels of shed AXL (sAXL) and GAS6 in both PCC and stroma regions, they found a significant positive correlation between relative sAXL and GAS6 levels and lymph node metastasis. Given the extremely high binding affinity (Kd of 33 pM) between AXL and GAS6, they validated that sAXL could act as a high-affinity decoy receptor to neutralize GAS6 and thereby reduce AXL signaling.
The team then explored the phenotypic effects of MMP-mediated AXL signaling on PDAC tumour growth. Adopting the R428 AXL kinase inhibitor that entered into a phase II clinical trial for PDAC treatment, they found a notable synergistic effect between R428 and MMP inhibitor BB-94 in patients-derived organoids (PDOs). This effect was validated in vivo using PDOs-derived xenograft models. The combined administration of R428 and BB-94 significantly reduced tumour growth in an orthotopic mouse model compared to either treatment alone. Collectively, they demonstrated that sAXL could act as a decoy receptor for GAS6 to attenuate intercellular AXL signaling and potentially regulate PDAC tumour growth and metastasis. Measuring AXL shedding and GAS6 levels may help identify patients who could benefit more from AXL inhibitor therapeutic treatment.
Overall, this study provides a comprehensive intercellular signalling landscape with an interactive website interface (see TMExplorer link below) for functional and translational research on PDAC. TMEPro is widely applicable for studying other cancer types, and the proteomic resources are of broad interest to the community, especially when testable biomarkers and clinical vulnerabilities are not identifiable through genomic assessment alone.
Scientist Peiwu Huang and Research Assistant Professors Weina Gao and Changying Fu, all from SUSTech, along with Professor Min Wang from Huazhong University of Science and Technology (HUST) and postdoctoral fellow Yunguang Li from the Center for Excellence in Molecular Cell Science (CEMCS) of the Chinese Academy of Sciences (CAS), are the co-first authors of the paper.
Professor Ruijun Tian, Professor Renyi Qin from HUST, Researcher Dong Gao from CEMCS, CAS, and Dr. Yu Shi from the Salk Institute for Biological Studies (Salk Institute) are the corresponding authors. Other contributors to this work include Tony Hunter from the Salk Institute, Research Assistant An He, Ph.D. student Yuan Li, and postdoctoral fellow Qian Kong. SUSTech is the first affiliated institution of the paper.
Paper link: https://www.nature.com/articles/s41586-024-08225-y
TMExplorer link: http://bioinfo.chem.sustech.edu.cn/TMExplorer_Beta/user-login.jsp
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo ByDepartment of Chemistry