Electrochromic oxides, known for their ability to dynamically change visible and near-infrared spectra, hold great potential for applications such as smart windows, displays, and camouflage. Although these applications depend on the optical properties of electrochromic oxides, the origin of the optical absorption is still unclear.

The research group led by Associate Professor Rui-Tao Wen from the Department of Materials Science and Engineering at the Southern University of Science and Technology (SUSTech) has made new progress in understanding these electrochromic materials.
Under the framework of polaron theory, they found that the coloration of all amorphous cathodic electrochromic oxides can be described by a combination of small- and bi-polarons. This breakthrough reveals the origin of optical absorption in these oxides and paves the way for achieving dual-band regulation in such materials.
This work was published in Applied Physics Reviews with the title “Polaron hopping induced dual-band absorption in all amorphous cathodic electrochromic oxides”, and was also selected as a Featured Article in the journal.
The researchers established an optical absorption model based on polaron hopping, according to the valence state variations of metal elements in oxides. Their study systematically analyzed the optical absorption in traditional cathode electrochromic oxides (WO3, MoO3, Ta2O5, Nb2O5, TiO2) and so-called bipolar electrochromic V2O5. By comparing the optical density of amorphous electrochromic oxides with the polaron hopping model, they identified two prominent absorption peaks in the spectrum. These peaks originated from the charge transfer of small polarons and bipolarons, the transfer of single charge between Mn+ and M(n-1)+ and the transfer transition of double charge between Mn+ and M(n-2)+.
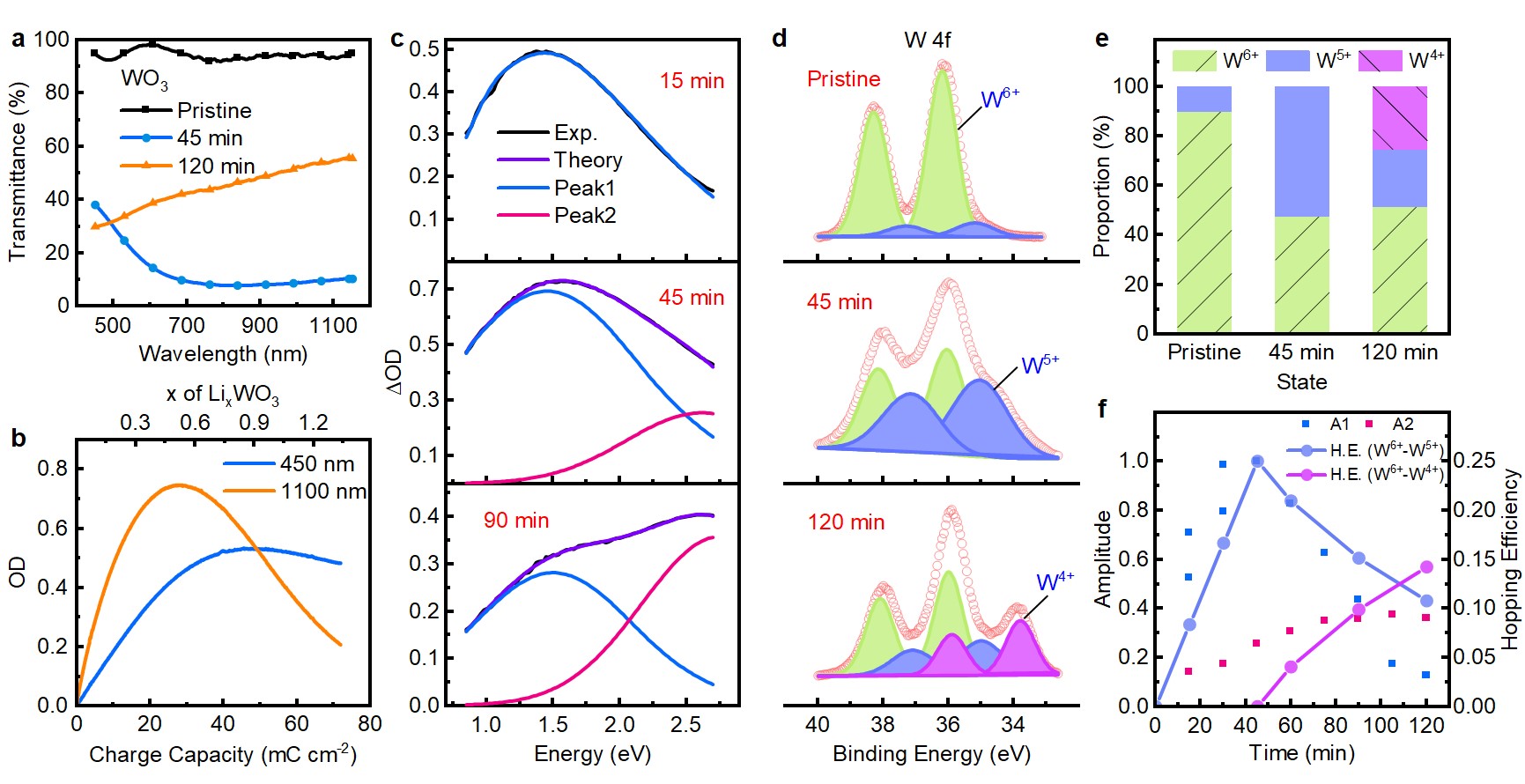
Figure 1. Polaron hopping induces dual-band absorption of WO3
They further proved that small polaron and bipolaron hopping can lead to dual-band modulation, demonstrating that visible and near-infrared light can be controlled separately. Specifically, in the initial stage of charge insertion, the small polaron hopping between Mn+ and M(n-1)+ leads to near-infrared light absorption. Further charge insertion leads to the formation of M(n-2)+ (except TiO2), which promotes the bipolaron hopping between Mn+ and M(n-2)+ sites, thereby triggering light absorption in the short wavelength range.
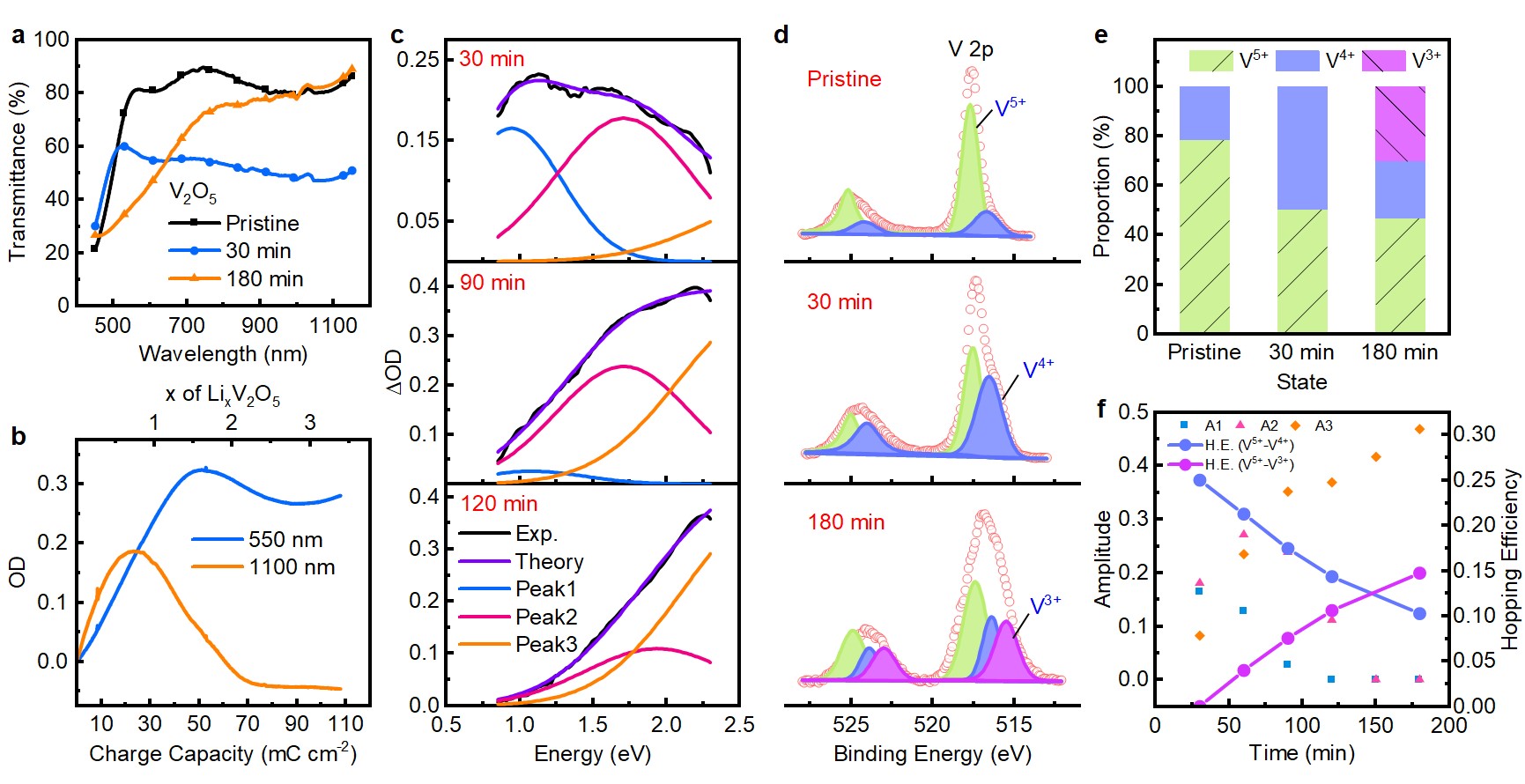
Figure 2. Polaron hopping induces multi-band absorption of V2O5
The dual-band modulation induced by small polaron and bipolaron hopping was more obvious in V2O5. Therefore, within the framework of polaron hopping, V2O5 shares the same electrochromism with other cathodic electrochromic oxides, and should be classified as cathodic oxides rather than a bipolar one in the traditional view. This result offers a deep understanding of the electrochromism of cathodic oxides and provides a basis for the development of electrochromic devices with dual-band modulation.
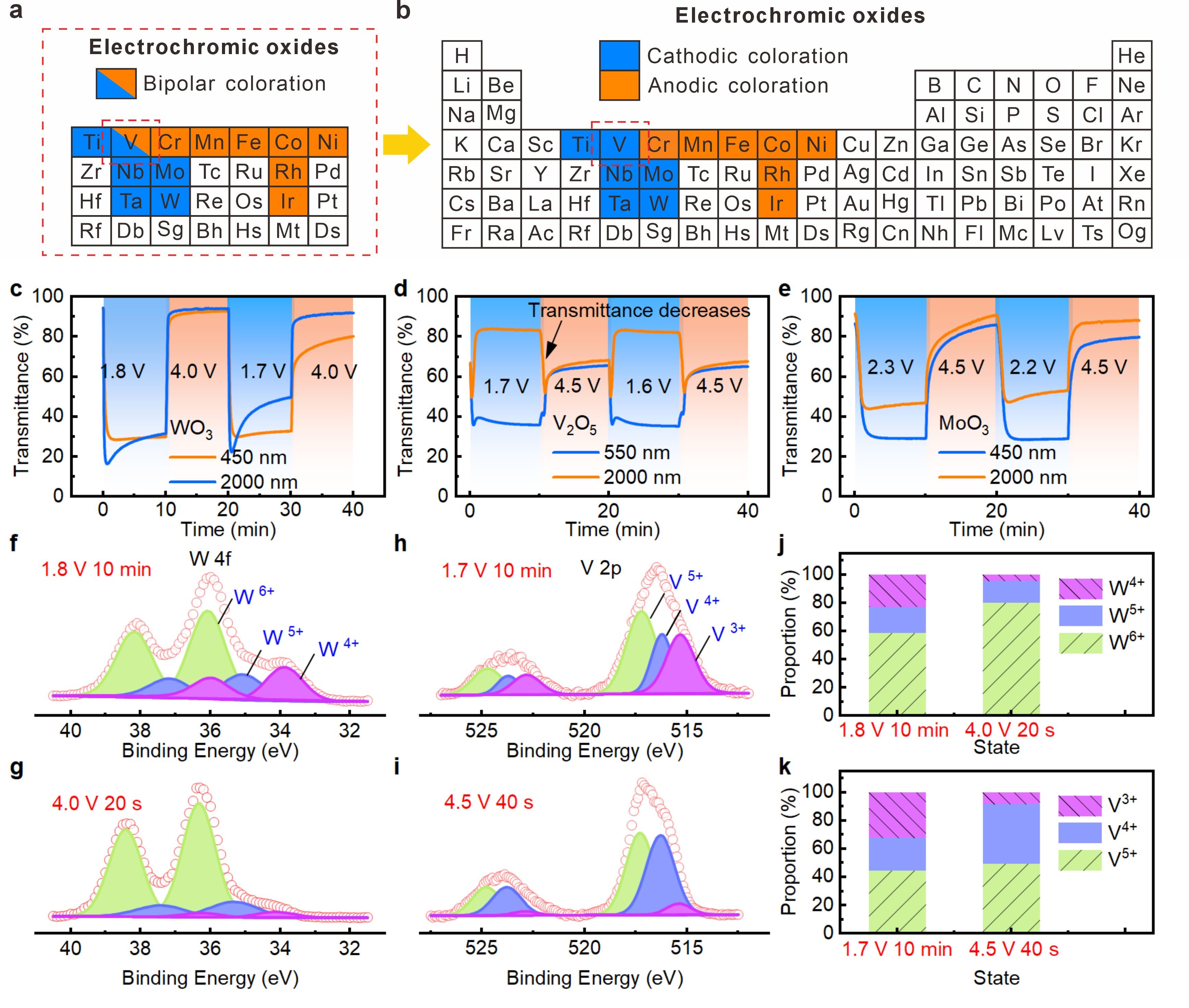
Figure 3. Updated classification of electrochromic cathodic oxides
Dr. Renfu Zhang is the first author of the paper, with Associate Professor Rui-Tao Wen serving as the corresponding author. Other authors from SUSTech include master’s student Yin Menghan, doctoral student Shao Peipei, and Dr. Qingjiao Huang, alongside Professor Gunnar Niklasson from Uppsala University.
Paper link: https://doi.org/10.1063/5.0244549
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo ByDepartment of Materials Science and Engineering