GTPases are a class of highly conserved regulatory proteins that play essential roles in various biological activities such as signal transduction, protein synthesis, and ribosome biogenesis. OLA1/YchF, a member of the TRAFAC family of P-loop GTPases, possesses a unique (N/T)(M/L/V)xE amino acid sequence that causes it to lose guanosine triphosphate (GTP) specificity, preferentially hydrolyzing adenosine triphosphate (ATP) rather than GTP. This distinctive ATP hydrolysis property has drawn significant attention from researchers.
OLA1/YchF is a multifunctional protein involved in various cellular stress response pathways, including heat shock, integrin stress, cell adhesion, and antioxidant responses. It is also linked to the progression of several cancers, such as breast cancer, lung cancer, and hepatocellular carcinoma. OLA1 is highly expressed in most cancers and is considered a potential cancer biomarker and therapeutic target.
Recent evidence suggests that OLA1/YchF plays a vital role in regulating the translation of specific mRNAs, such as leaderless mRNAs encoding stress response proteins that maintain cell survival. OLA1/YchF binds to the ribosome, a phenomenon observed in E. coli, yeast, trypanosomes, plants, and human cells. However, many unanswered questions remain regarding the molecular details of ribosomal binding and translation regulation, as well as the precise role of OLA1/YchF in these processes.
The concept for the design was inspired by the film Ne Zha 2
A research team led by Assistant Professor Fuxing Zeng from the Department of Systems Biology, School of Life Sciences at the Southern University of Science and Technology (SUSTech) has revealed a key mechanism by which OLA1/YchF regulates translation. Their work uncovers that the OLA1/YchF protein not only binds to the large subunit of the ribosome, but also promotes the efficient translation of mRNA rich in aspartic acid (D) and glutamic acid (E). Their findings provide a new perspective for understanding ribosomal function.
Their paper, titled “Conserved GTPase OLA1 promotes efficient translation on D/E-rich mRNA”, has been published in the journal Nature Communications.
In this study, Fuxing Zeng’s team successfully solved the structure of the YchF-50S ribosomal subunit complex (Figure 1). They found that YchF mainly interacts with the ribosomal 50S subunit through three key sites. The first binding site involves the helical domain forming electrostatic interactions with the H62 helix of the 50S subunit via two conserved lysine residues. The second binding site involves interaction between the helical domain and the bL19 protein, with the third binding site involving the ATPase domain interacting with the uL14 protein.
Given that YchF binds at a very specific position on the 50S subunit, located at the interface between the 50S large subunit and the 30S small subunit, the research team sought to understand the important function this crucial position might serve. After a series of structural analyses and biochemical experiments, they discovered that the binding of YchF to this site prevents the ribosomes from reassociation.
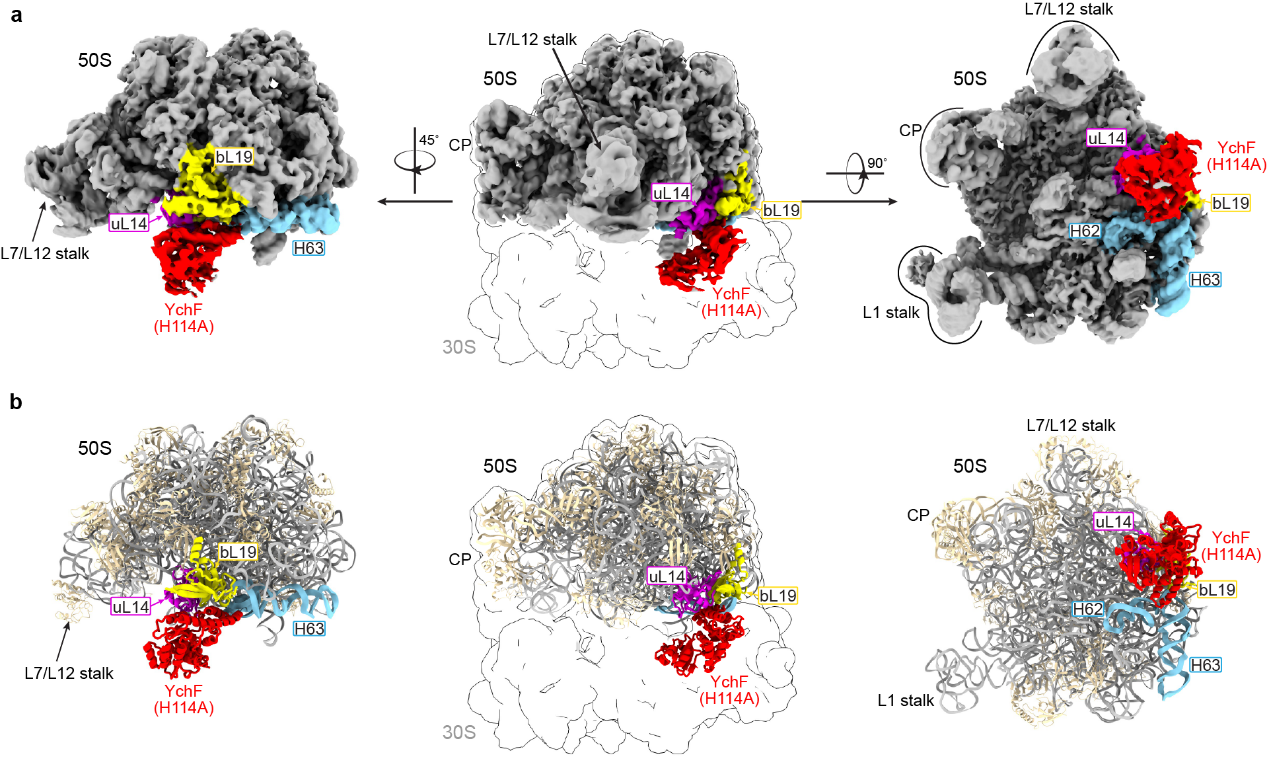
Figure 1. Cryo-EM structure of YchF bound to the ribosomal 50S subunit
To further explore the function of OLA1/YchF binding to the ribosome, the researchers knocked out the OLA1 gene and performed biochemical experiments and Ribo-seq high-throughput sequencing analysis (Figure 2). The analysis of sequencing results and biochemical validation confirmed that OLA1/YchF promotes the translation of D/E-rich mRNAs. The genes regulated by OLA1/YchF are primarily ribosomal and translation-related. By regulating the mRNA levels of these proteins, OLA1/YchF helps control the cell’s response to stressful environments and the development of tumors, providing an important theoretical basis for the development of new cancer treatment strategies.
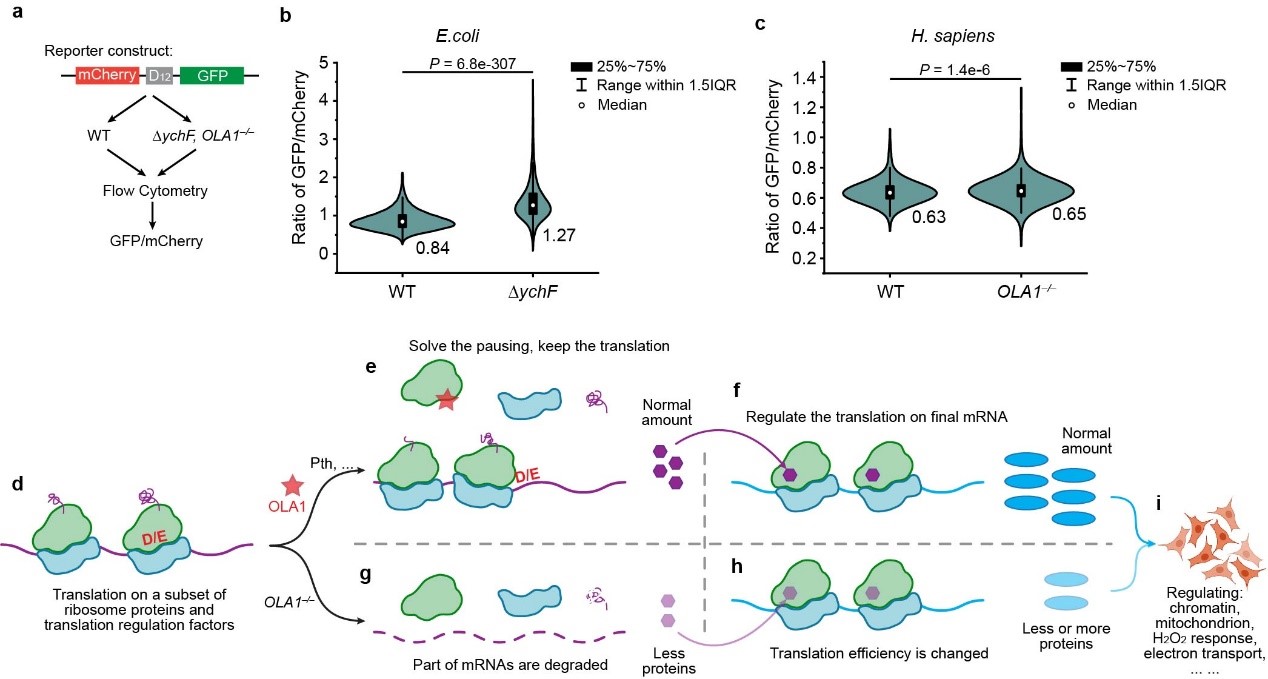
Figure 2. Model of translation regulation mediated by OLA1/YchF
Ph.D. student Ting Yu and Research Assistant Xin Li from the School of Life Sciences at SUSTech are the co-first authors of the paper. Assistant Professor Fuxing Zeng is the corresponding author, with SUSTech serving as the first affiliated institution. Other contributors to this work include Ph.D. students Wanlin Dong and Qingrong Li, along with master’s students Qixin Zhou and Zisuo Du.
Paper link: https://www.nature.com/articles/s41467-025-56797-8
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yilin ZHOU
Photo ByDepartment of Systems Biology