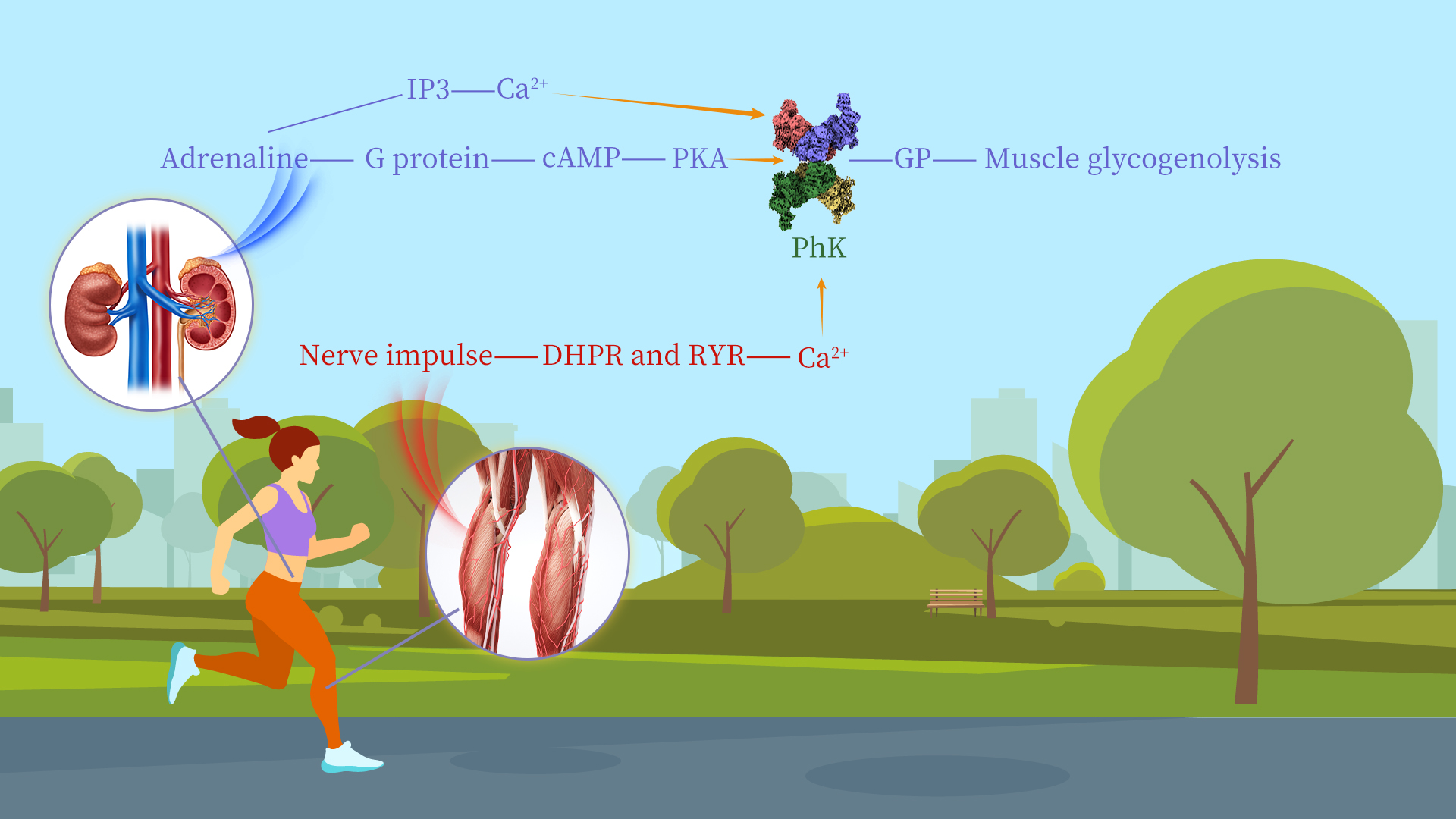
Glycogen is the main storage form of glucose in animals and humans, which plays a crucial role in regulating blood sugar levels and ensuring a steady supply of energy to the body. The breakdown of glycogen in tissues is controlled through the action of several protein kinases and phosphatases. Phosphorylase kinase (PhK) plays a key role in the cascade system for regulating glycogen metabolism. It phosphorylates a single serine residue of glycogen phosphorylase (GP), catalyzing the conversion of inactive phosphorylase b (GPb) into active phosphorylase a (GPa). GPa catalyzes the degradation of glycogen, ultimately generating energy to support a broad range of cellular activities in most tissues.
PhK is recognized as one of the largest and most complex protein kinases known. It comprises four subunits—α, β, γ, and δ—assembled into a 1.3 MDa heterohexadecameric α4β4γ4δ4 complex. The activity of PhK is regulated by hormonal and neural signals. When these signals are transmitted to PhK, its α and β subunits undergo phosphorylation by cyclic AMP (cAMP)-dependent protein kinase A (PKA), leading to partial activation of PhK. On the other hand, the δ subunit, which functions as the endogenous molecule calmodulin (CaM), mediates the regulation of PhK activity by calcium ions (Ca2+) triggered by neuronal stimulation. Notably, dysfunctions in PhK are closely associated with glycogen storage diseases and certain cancers.
A research team headed by Associate Professor Kaige Yan from the Departments of Chemistry and Biology, School of Life Sciences at the Southern University of Science and Technology (SUSTech) has uncovered a key mechanism underlying the regulation of PhK activity and substrate binding. Their research elucidates the high-resolution structures of PhK under phosphorylation and Ca2+-bound conditions, deepening our understanding of how hormonal and neural signals regulate PhK. This structural knowledge will offer crucial insights for the development of PhK-targeted therapies aimed at modulating glucose regulation and treating disorders related to glycogen metabolism.
Their work, entitled “Molecular basis for the regulation of human phosphorylase kinase by phosphorylation and Ca2+”, has been published in the journal Nature Communications.
In this study, Kaige Yan’s team successfully determined the high-resolution cryo-EM structures of human PhK in its inactive state and in the phosphorylation/Ca²⁺-activated state. Through these structural investigations, the team revealed the interactions and relative positions of the four subunits (α, β, γ, δ) within the PhK complex. From the overall structure, the front view of PhK resembles a butterfly, while its side views present a chalice-like and cuboid shape. The β subunit constitutes the body of the butterfly, whereas the αγδ heterotrimer forms the wings, with the two connected via the α subunit. This distribution pattern enables the catalytic subunit γ to be simultaneously regulated by the regulatory subunits α, β, and δ.
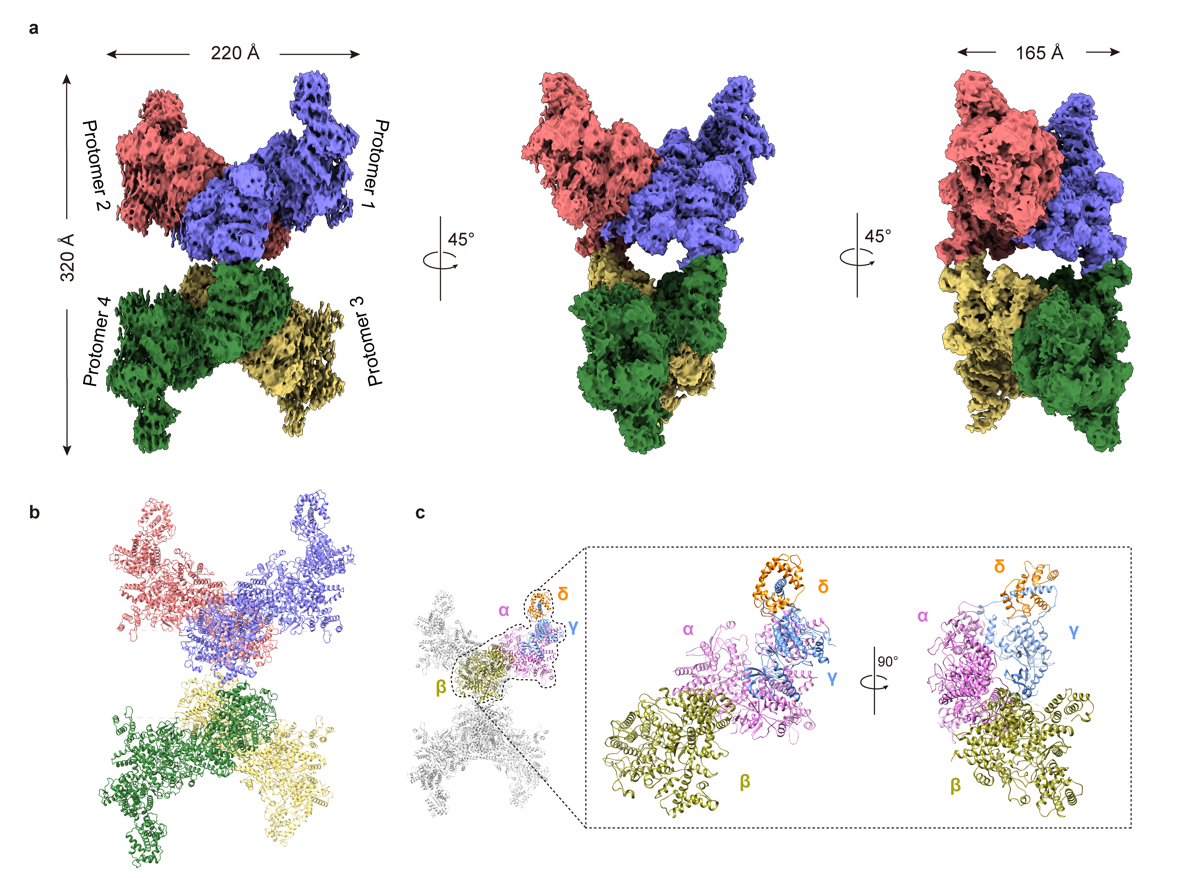
Figure 1. Cryo-EM structure of human PhK
Further analysis revealed that PKA primarily phosphorylates the Ser1018 residue of the α subunit and the Ser27 and Ser700 residues of the β subunit, causing the four protomers to move toward the center and thereby destabilizing the γ-N lobe of the catalytic subunit. Ca²⁺ binding to the δ subunit (CaM) results in the sliding of the δ subunit along the γ-CBD (CaM binding domain) region, destabilizing the γ-C lobe. Additionally, the research team employed cross-linking mass spectrometry to analyze the different binding modes of PhK with its substrate in the inactive and activated states. In the inactive state, the substrate interacts with the α and β subunits. Upon phosphorylation/Ca²⁺ activation, the stability of the CKD (catalytic kinase domain) region of the δ subunit decreases, and the proximity of the substrate GP further induces a conformational flip of the CKD domain, exposing the active center and thereby enabling catalytic activity. This study provides valuable insights into the regulatory mechanisms of PhK and offers a scientific basis for treating glycogen storage diseases caused by PhK abnormalities.
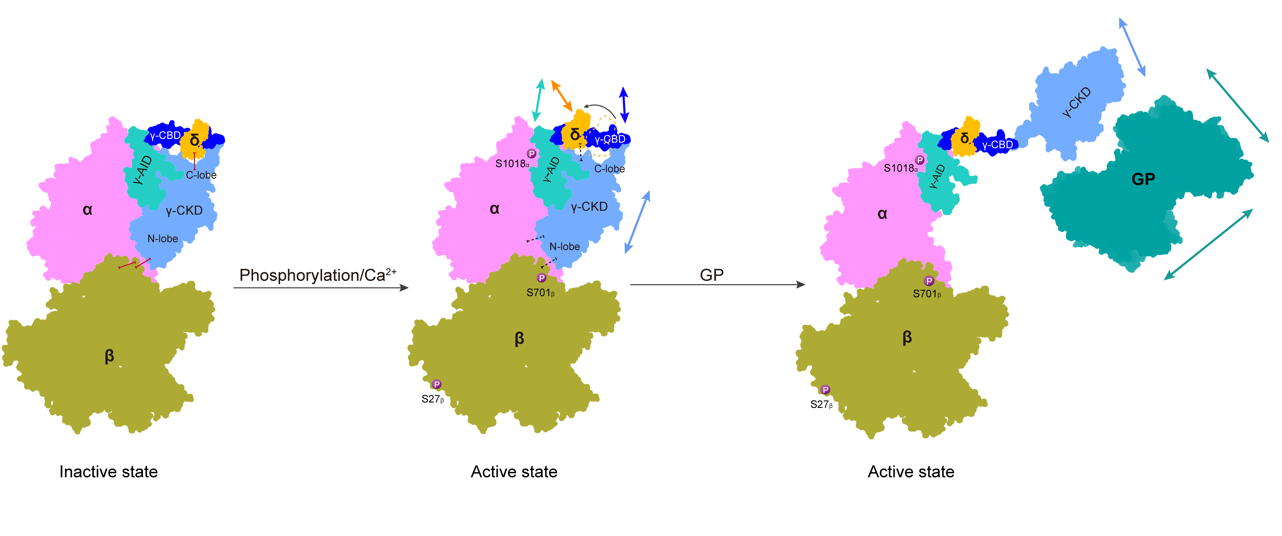
Figure 2. Proposed regulation model of hPhK activity
Research Assistant Professor Ruifang Ma and Ph.D. candidate Bowen Du from the School of Life Sciences at SUSTech are the first authors of the paper. Ph. D. candidate Chen Shi from Zhejiang University is the co-first author, with Assistant Professor Kaige Yan and Professor Yong Wang from Zhejiang University serving as the corresponding authors. Additional contributors to this research include doctoral student Lei Wang, Assistant Professor Fuxing Zeng, doctoral student Jie Han, and master’s student Huiyi Guan.
Paper link: https://www.nature.com/articles/s41467-025-58363-8
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yuwen ZENG
Photo BySchool of Life Sciences