Autism spectrum disorder (ASD) is a complex neurodevelopmental disorder characterized by social novelty deficits and communication impairments. Individuals with a deletion in the 16p11.2 chromosomal region have an increased risk of developing ASD. Previous studies have shown that the 16p11.2 chromosomal region is associated with multiple neurodevelopmental disorders, including autism, intellectual disabilities, and schizophrenia.

A research group led by Professor Sheng-Tao Hou from the School of Life Sciences at the Southern University of Science and Technology (SUSTech) has recently published a study that identifies CD47, a key protein in regulating immune responses, as a crucial factor in enhancing microglial phagocytosis of synapses in the brain. The work demonstrates that CD47 downregulates excitatory synaptic transmission and improves social novelty deficits in the autism mouse model. Using a 16p11.2 deletion mouse model, the team reveals how abnormalities in neural circuitry and synaptic transmission are related to ASD symptoms. Notably, the findings uncover the neuroimmune regulatory role of CD47, providing new insights for the treatment of neurodevelopmental disorders associated with autism.
Their paper, titled “The don’t eat me signal CD47 is associated with microglial phagocytosis defects and autism-like behaviors in 16p11.2 deletion mice”, has been published in the Proceedings of the National Academy of Sciences (PNAS).
In this study, the team found that 16p11.2 deletion mice exhibited autism-like behavioral characteristics, including impaired cognitive memory and social novelty abilities. In the prefrontal cortex (PFC) region of 16p11.2 deletion mice, the number of microglia remained unchanged, but their phagocytic function was reduced, leading to an increase in synapse numbers. By applying CD47 antibodies or CD47 shRNA, they found that they could enhance microglial phagocytosis and reduce synapse numbers. This suggests that CD47 protein may serve as a potential therapeutic target to improve autism phenotypes.
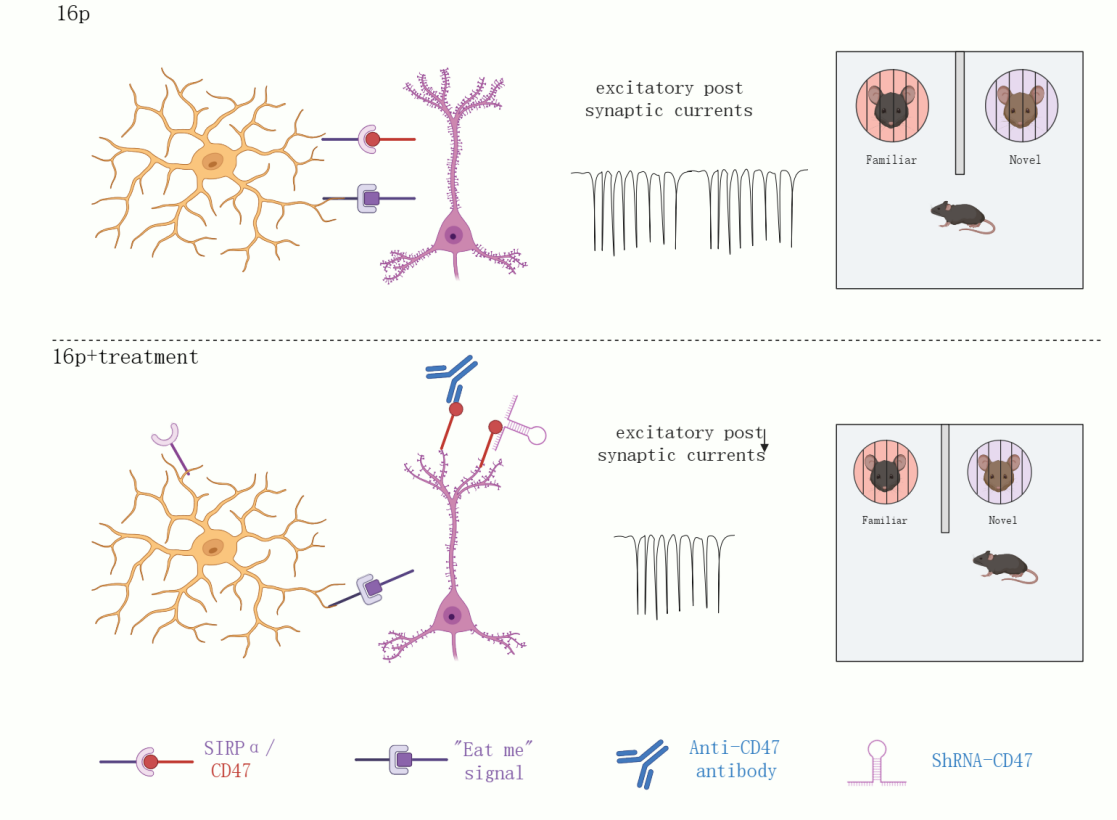
Figure 1. Experimental design and mechanism of CD47
Further analysis confirmed that excitatory synaptic transmission in the PFC was significantly increased in 16p11.2 deletion mice, likely due to reduced microglial phagocytic function. These mice also showed marked deficits in social novelty behavior.
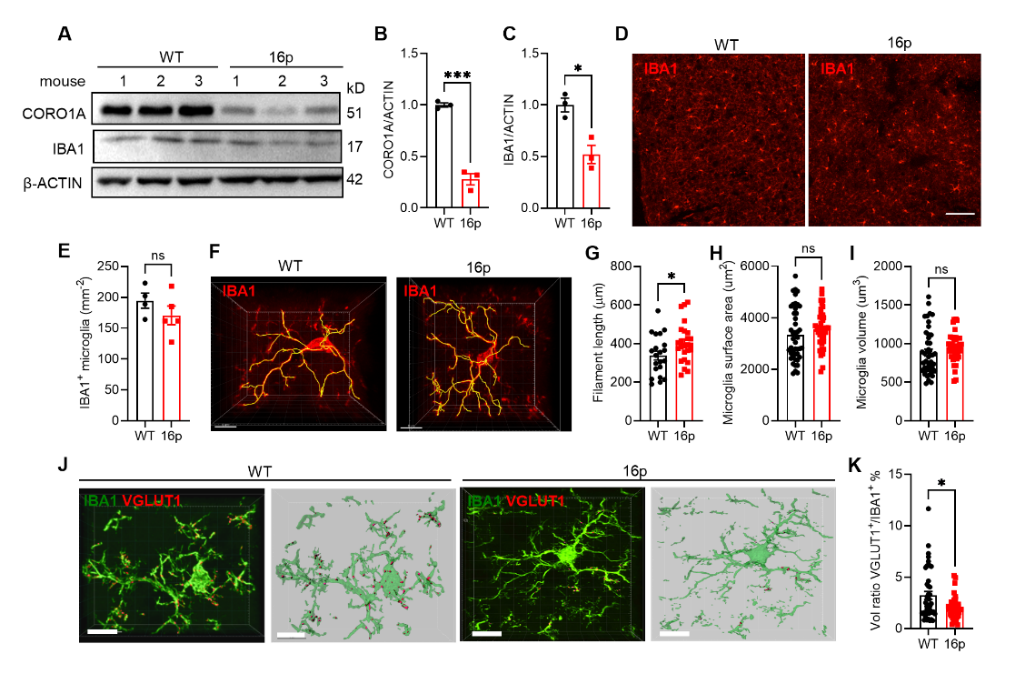
Figure 2. Reduced microglia-dependent synaptic pruning in 16p11.2 deletion mice
CD47, known as a “don’t eat me” signal, was significantly elevated in 16p11.2 deletion mice and showed a significant negative correlation with social novelty ability in mice. To further demonstrate that CD47 is involved in the behavioral abnormalities of 16p11.2 deletion mice, the researchers blocked its signaling pathway using CD47 antibody and found that CD47 antibody could enhance microglial phagocytosis of synapses, thereby downregulating excitatory synaptic transmission in the PFC region. Similarly, using CD47 shRNA virus also downregulated excitatory synaptic transmission and improved social novelty deficits in 16p11.2 deletion mice.
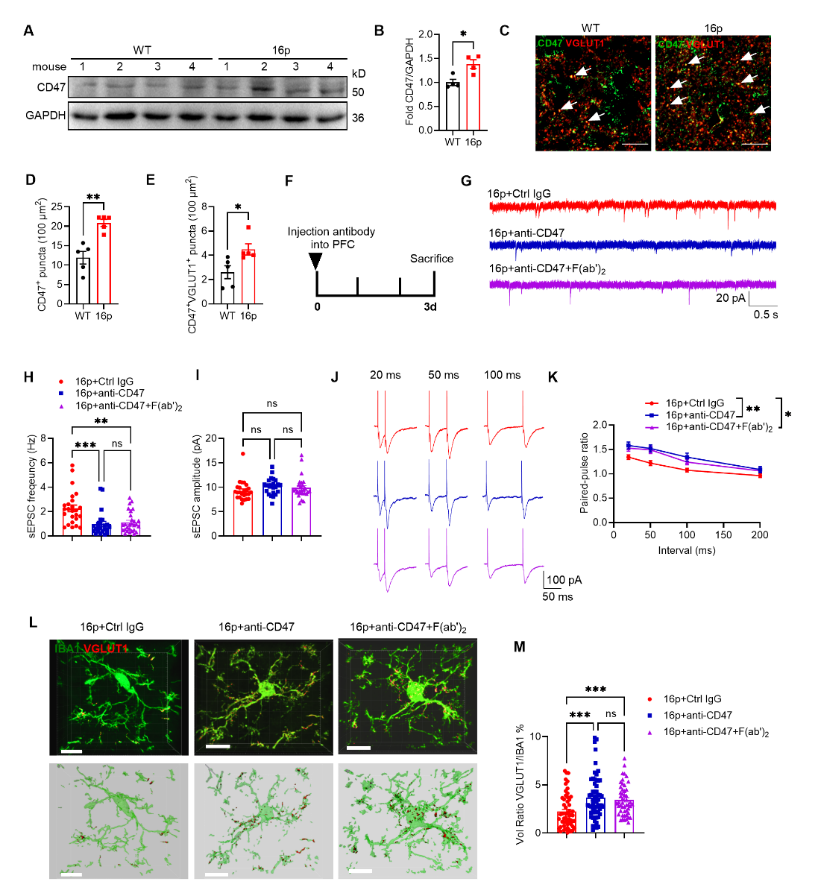
Figure 3. Blocking CD47 with specific antibodies reduces excitatory synaptic transmission in 16p11.2 deletion mice
This study confirms that CD47 level is abnormally elevated in autism, and blocking its signaling pathway can enhance microglial phagocytosis, downregulate excitatory synaptic transmission, and alleviate social novelty deficits in an autism mouse model. Blocking CD47 holds promise as a clinically viable therapeutic approach for autism.
Research Assistant Professor Jun Ju from Professor Sheng-Tao Hou’s group is the first author of the paper. Professor Hou is the corresponding author, with SUSTech serving as the primary institution. Other contributors to this study include team members Yifan Pan, Xinyi Yang, Xuanyi Li, Jinghong Chen, and Shiyu Wu deeply participated in the research.
Paper link: www.pnas.org/doi/10.1073/pnas.2411080122
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yilin ZHOU
Photo BySchool of Life Sciences