ATP-binding cassette (ABC) transporters are a class of membrane proteins widely found across all domains of life. These proteins harness the energy from ATP hydrolysis to drive the translocation of a diverse range of substrates across membranes. In plants, ABC transporters play essential roles in nutrient uptake, the transport of hormones (such as auxin and abscisic acid), and the movement of secondary metabolites. As key regulators of plant growth, development, and stress responses, they present promising targets for enhancing crop resilience and yield.
Recent advances in cryo-electron microscopy (cryo-EM) have gradually unveiled the conformational dynamics and substrate recognition mechanisms of plant ABC transporters. Notably, plant ABCB1 and ABCB19 have been implicated in the transport of brassinosteroids (BRs). However, the critical conformations involved in substrate translocation, as well as the molecular basis of their regulation by small molecules, have remained elusive.

A research team led by Chair Professor Maofu Liao from the Department of Chemical Biology, School of Life Sciences at the Southern University of Science and Technology (SUSTech) has now elucidated the conformational cycle of the plant ABCB19 transporter in a lipid environment. For the first time, they have revealed the mechanism of its inhibition by small molecules.
Their study, entitled “Conformational cycle and small-molecule inhibition mechanism of a plant ABCB transporter in lipid membranes”, was published in Science Advances on June 13, 2025.
The researchers reconstituted ABCB19 into lipid nanodiscs that mimic physiological membrane environments and found that its ATPase activity increased more than 20-fold compared to that in detergent micelles, underscoring the critical role of lipid membranes in supporting transporter function. Upon the addition of brassinolide (BL), an active BR compound, ABCB19 activity was further enhanced by approximately threefold. Intriguingly, high concentrations of BL produced an inhibitory effect, revealing a bidirectional regulatory mechanism that is dependent on hormone concentration.
Using single-particle cryo-EM, the team resolved multiple structural states of ABCB19, including its apo form, BL-bound state, vanadate-trapped closed conformation, and a state bound to a small-molecule inhibitor, thereby reconstructing the entire transport cycle.
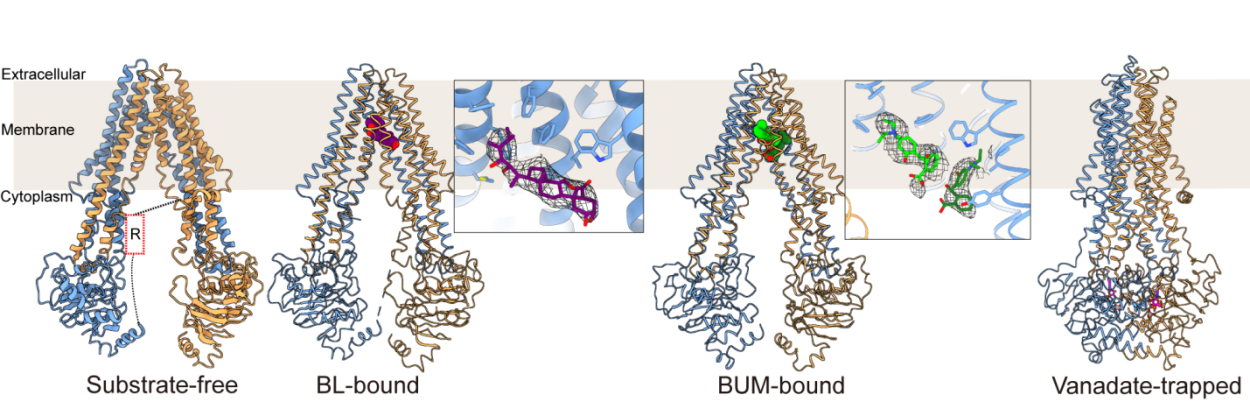
Figure 1. 3D structure of ABCB19
Biochemical and structural analyses revealed that vanadate-trapped closure of ABCB19 exhibits strong temperature dependence, explaining previous difficulties in obtaining this conformation at lower temperatures.
Given the central role of ABCB transporters in hormone translocation, developing selective inhibitors is of significant interest. The researchers determined the cryo-EM structure of ABCB19 bound to the ABCB-specific inhibitor BUM (2-[4-(diethylamino)-2-hydroxybenzoyl]benzoic acid). The structure showed that two BUM molecules occupy a hydrophobic pocket that substantially overlaps with the BL-binding site, locking ABCB19 in an inward-facing conformation with separated nucleotide-binding domains (NBDs) and thereby halting its transport activity. Comprehensive mutagenesis and functional assays further identified key residues involved in both substrate and inhibitor recognition.
This study provides a systematic analysis of the conformational transitions and functional mechanisms of the plant ABC transporter ABCB19 within a near-physiological lipid membrane environment. It represents the first full reconstruction of the transporter’s activity cycle and its inhibition by small molecules. These findings offer novel insights into plant hormone transport and establish a structural and mechanistic framework for the rational design of plant growth regulators.
Dr. Yong Liu is the first author of the paper, with Chair Professor Maofu Liao serving as the corresponding author.
Paper link: https://www.science.org/doi/10.1126/sciadv.adv9721
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yilin ZHOU
Photo ByYan QIU