Recently the group of Chair Prof. Xumu Zhang from the Department of Chemistry published significant work in the journal of J. Am. Chem. Soc. ( IF = 13.858)on asymmetric reductive amination of simple ketones. This work was completed by Dr. Xuefeng Tan (the first author), Shuang Gao (MS), Weijun Zeng (BS), Shan Xin (MS). Mr. Weijun Zeng became an undergraduate student at SUSTech in 2014. Qin Yin, a research Professor from Academy for Advanced Interdisciplinary Studies, and Prof. Xumu Zhang from the Department of Chemistry were corresponding authors.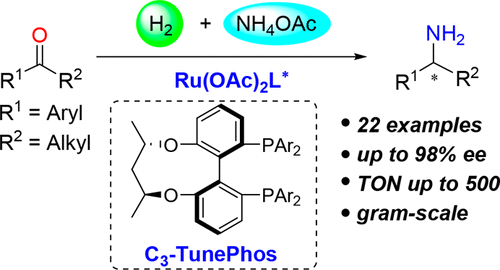
Chiral amines are prevalent structural units in a large number of natural products and pharmaceutical drug molecules. Therefore, efficient synthetic routes toward chiral amines have attracted tremendous attention. Transition metal (TM)-catalyzed reductive amination represents one of the simplest and efficient strategies by directly converting carbonyl compounds into amines. Showed below are some representative drugs containing chiral amine moisty:

There are two main challenges in TM-catalyzed reductive amination. Firstly, the amine source and the yielding product, which is also amine, will cause poisoning through the coordination of amine to metal. Secondly, the incompatibility of transition-metal hydrides with ketones will cause severe side effects. Special amine sources such as anilines are frequently used to solve these two challenges, since they can efficiently condense with ketones to furnish imines and the resulting reduced chiral amine products possess very weak coordinating ability toward metals, thus having limited poisoning effect. An additional synthetic operation to remove the aryl or benzyl substituents is thus required to liberate the synthetically more versatile primary amine. Notably, direct synthesis of the chiral primary amine from simple ketones utilizing reductive amination is rarely studied, and only one example involving transfer hydrogenation has been reported so far.
In this work, a ruthenium/C3-TunePhos catalytic system has been identified for highly efficient direct reductive amination of simple ketones. The strategy makes use of ammonium acetate as the amine source and H2 as the reductant to efficiently furnish valuable chiral primary amines with high selectivity and broad substrate scope. Remarkably, the ligand C3-TunePhos utilized for this work was originally developed by the same group. Showed below is the substrate scope of this work:

To highlight the utility of this method, key intermediates of three drugs Tecalcet hydrochloride, Cinacalcet and Rivastigmine can be accessed easily in gram scale from commercially available ketones. Showed below are scalable syntheses of drug intermediates:

The authors greatly acknowledge SUSTech for financial support. In addition, this research was supported by Shenzhen Science and Technology Innovation Committee and National Natural Science Foundation of China.
Proofread ByChris Edwards
Photo ByDepartment of Chemistry