Ground-breaking research from the Second Affiliated Hospital of SUSTech offers a preliminary take on the use of the plasma cells of patients of the novel coronavirus (COVID-19) for the recovery of critically ill patients. Convalescent plasma therapy is the use of antibodies from the blood of a person who has recovered from a specific disease and then transferring them into an infected person.
It is the first global report on the use of convalescent plasma treatment for severe COVID-19 patients. In an editorial, the Journal of the American Medical Association said that many institutions around the world should encourage discharged patients to donate plasma as part of a large-scale randomized clinical trial, following the success of this preliminary study.
On March 27, the Second Affiliated Hospital of Southern University of Science and Technology (SUSTech), also known as the Shenzhen Third People’s Hospital, published a paper in the high-impact academic journal, Journal of the American Medical Association (IF = 51.273). The paper was titled, “Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma,” and discussed the scholar’s initial clinical experience of using convalescent plasma therapy to treat critically ill patients.
With COVID-19 spreading across the globe, there is a race to find a therapeutic drug or vaccine against COVID-19. There is significant literature that has shown the beneficial impact of convalescent plasma therapy on critically ill patients suffering from Ebola, SARS, or influenza. However, there was no such research on the efficacy of this therapy for COVID-19.
The Second Affiliated Hospital of SUSTech (Shenzhen Third People’s Hospital) and the National Clinical Research Center for Infectious Disease led the charge in this field, as they commenced their study on January 30.

Their study of five COVID-19 patients who were critically ill were infused with the plasma of recovered patients. Within three days, four of the five patients returned to a normal temperature range. The ratio of the SOFA and the PAO2 / FIO2 evaluation index (used to assess the injury to the lungs) increased significantly, indicating the treatment had a positive impact. After twelve days, the virus was negative in vivo, meaning that there were lower viral loads and higher COVID-19 specific antibodies.
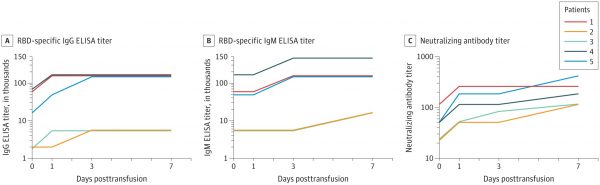
In this preliminary, non-control study of five critically-ill patients with COVID-19, it appears that convalescent plasma treatment offers an improvement in the clinical status of the patients. The researchers stress that it is impossible to make a definitive statement as to the potential effectiveness, and clinical trials are needed to assess the effectiveness of the treatment.
Professors Yingxia Liu, Zheng Zhang, and Lei Liu of Second Hospital Affiliated to SUSTech (Shenzhen Third People’s Hospital) were the co-correspondent authors. Drs Shen Chuanguang, Zhaoqin Wang, Zhao Fang, and Yang Yang were all co-first authors. Additional contributions came from the Laboratory of Protein Engineering and Vaccines, Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences (CAS).
This work was supported by the National Science and Technology Major Project, Sanming Project of Medicine in Shenzhen, China Postdoctoral Science Foundation, Shenzhen Science and Technology Research and Development Project, National Natural Science Foundation of China, Shenzhen Science and Technology Research and Development Project, and The Key Technology R&D Program of Tianjin.
Paper: https://jamanetwork.com/journals/jama/fullarticle/2763983
Editorial: https://jamanetwork.com/journals/jama/fullarticle/2763982
Proofread ByXia Yingying
Photo BySecond Affiliated Hospital of SUSTech (Shenzhen Third People's Hospital), Qiu Yan