Noncovalent interactions are ubiquitous in nature and are responsible for the precision control in enzyme catalysis via the cooperation of multiple active sites. Inspired by this principle, noncovalent interaction-assisted transition metal catalysis has emerged recently as a powerful tool and has attracted intense interest.
However, it is still highly desirable to develop efficient and operationally convenient ligands along this line with new structural motifs.
Based on hydrogen bonding and ion-pairing interactions, Prof. Xumu Zhang’s team developed a series of noncovalent interaction-assisted chiral ferrocenyl phosphine ligands. This catalytic mode can achieve transition metal-catalyzed asymmetric hydrogenation and other transformations with remarkable reactivity and selectivity.
Recently, Prof. Xumu Zhang and his (former) group members team published a review article of recent advances and new strategies in asymmetric catalysis on Accounts of Chemical Research (IF = 20.8), entitled “Noncovalent Interaction-Assisted Ferrocenyl Phosphine Ligands in Asymmetric Catalysis.”

Hydrogen bonding has been widely observed in biosynthesis and enzyme catalysis. It plays an important role in molecular recognition, supramolecular chemistry, and small molecule catalysis. A new concept of organocatalysis emerged in recent two decades. Hydrogen bonding is the keystone for thiourea catalysis or phosphoric acid catalysis. With high turnover numbers and excellent enantioselectivity, transition metal catalysis is the arts in academia and finds its merit in industrial application. Among many successful transition metal-catalyzed reactions, homogeneous hydrogenation has been serving the synthetic communities for many years, both in academia and in industry. The success of secondary interaction and hydrogen bonding offers us an alternative: the combination of hydrogen bonding and steric hindrance helps create a chiral environment in which substrates could be reduced efficiently.
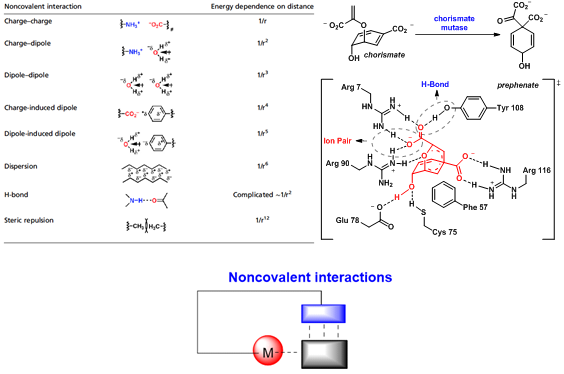
Guided by this rationale, Prof. Xumu Zhang (Chemistry, Chair Professor) and his team members have synthesized a series of ferrocene-based bisphosphine ligand, incorporating hydrogen bond donors such as thiourea and amine. With Zhaophos, the catalyst and the substrates could be bridged via hydrogen bonding. Transition metal-catalyzed asymmetric catalysis such as hydrogenation and allylic alkylation has been enabled with high efficiency and stereoselectivity. With Wudaphos or SPO-WUdaphos, the ion pair between the ligand and the substrate enabled asymmetric hydrogenation of unsaturated carboxylic acids with high enantioselectivity. More than 30 papers have been published in this field for over seven years.
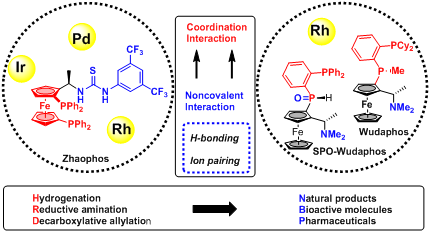
Dr. Qingyang Zhao (the inventor of Zhaophos, Prof. Xumu Zhang’s former student, currently an associate professor at Sun Yat-sen University), Dr. Caiyou Chen (the inventor of Wudaphos, Prof. Xumu Zhang’s former student, now a postdoc researcher in Caltech), Dr. Jialin Wen (Prof. Xumu Zhang’s former student, currently AAIS research assistant professor at SUSTech), Dr. Xiu-qin Dong (associate professor in Wuhan University) and Prof. Xumu Zhang are the co-authors of this review paper.
This project was financially supported by the National Natural Science Foundation of China, the Guangdong Provincial Key Laboratory of Catalysis, the Science, Technology, and Innovation Commission of, and the Fundamental Research Funds for the Central Universities.
Link to the paper: https://pubs.acs.org/doi/10.1021/acs.accounts.0c00347
Proofread ByEddy Salguero, Yingying XIA
Photo ByYan QIU