October 27, a research team led by Dr. Zhiyi Wei from the Department of Biology of SUSTech, Dr. Cheng Zhang from the University of Pittsburgh, and Dr. Fan Hao from the A*STAR Research Institute in Singapore published an article,entitled “Structural of human steroid 5α-reductase 2 with the anti-androgen drug finasteride” in Nature Communications.
It is the first time the three-dimensional structure of membrane-embedded eukaryotic steroid reductase was reported. This study uncovered the finasteride’s antiandrogenic effect for the treatment of alopecia and prostate hyperplasia.

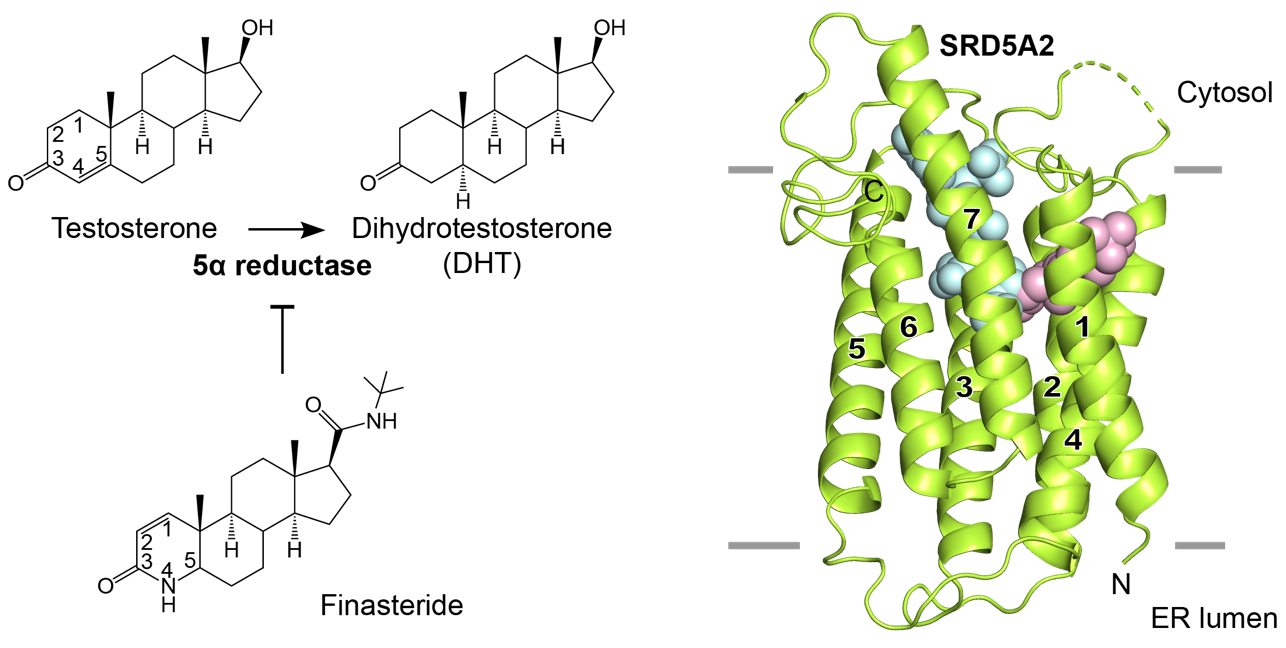
Figure 1. Structure of the complex formed by SRD5A2 (green), coenzyme NADPH (cyan), and inhibitor finasteride (pink).
The biosynthesis of sex hormones in the human body relies on steroid reductases. One of the steroid reductases, integral membrane steroid 5α-reductase 2 (SRD5A2), synthesizes the strongest androgen dihydrotestosterone (DHT) by catalyzing the reduction reaction of testosterone. DHT is essential for development and physiological activities. For instance, dihydrotestosterone controls the development of male sexual organs. Clinical studies have reported that a large number of mutations in the SRD5A2 enzyme result in low body levels of DHT and lead to male sexual characteristics deficiency.
On the other hand, an excessively high DHT level is the main culprit for prostate hyperplasia and hair loss. As a highly effective inhibitor of steroid reductase SRD5A2, finasteride is widely used to treat prostate hyperplasia and androgen-induced alopecia. Despite that SRD5A2 is an important enzyme in the human body, the mechanisms of SRD5A2 catalyzing the DHT synthesis and finasteride inhibiting the enzyme activity were largely unknown due to the lack of atomic-resolution structural information.
Recently, the international research team successfully solved the high-resolution crystal structure of the complex formed by SRD5A2 enzyme and finasteride. Structural analysis showed that SRD5A2 formed a unique seven-pass transmembrane fold, and the finasteride molecule occupied the binding pocket for the substrate testosterone molecule. Further analysis found that finasteride and the coenzyme factor NADPH are covalently linked to form a stable intermediate, which disrupts the transformation of coenzyme factor during the enzymatic catalysis process, thereby achieving a long-term inhibitory effect on the enzyme activity of SRD5A2. Combining structural biology, biochemistry, and molecular dynamics simulation technology, the team proposed a model of the catalytic mechanism of steroid reductase SRD5A2, explaining the effects of pathogenic mutations on enzyme structure and catalytic activity. This study promotes the understanding of various diseases caused by the imbalance of SRD5A2 activity in the human body. It provides valuable insights into the structure-based drug design in the future.
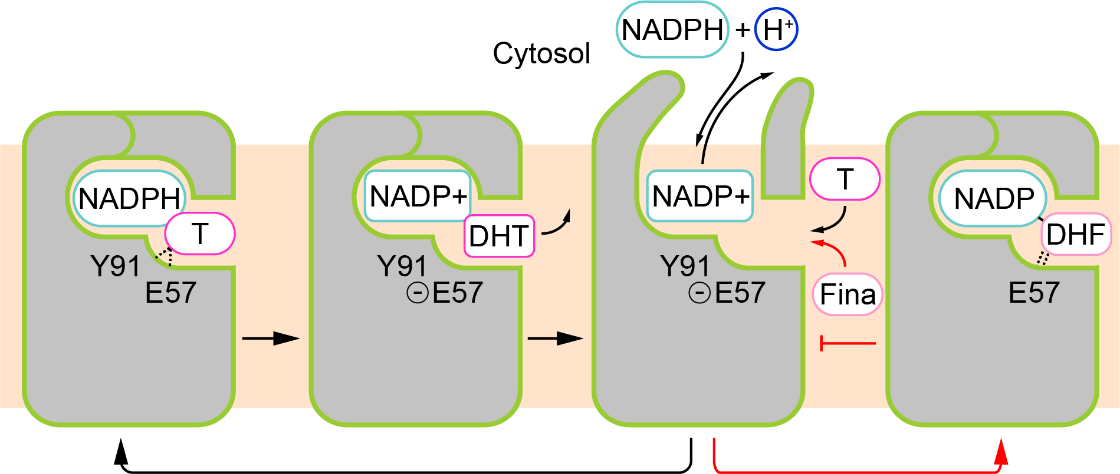
Figure 2. Model of SRD5A2 enzyme catalytic mechanism and finasteride inhibitory mechanism
This research was supported by the National Natural Science Foundation of China, Shenzhen-Hong Kong Institute of Brain Science, and the Core Research Facilities of Southern University of Science and Technology. Tencent AI Lab contributed to the study by providing a novel method for protein structure determination.
Paper link: http://www.nature.com/articles/s41467-020-19249-z
Profile of the author: http://faculty.sustech.edu.cn/weizy/en/
Proofread ByEddy Salguero, Yingying XIA
Photo ByYan QIU