The study of organelles’ dynamics and their interactions have been limited by optical diffraction. Recently, teams from the Chair Prof. Dayong Jin at SUSTech and Prof. Xi Peng (Peking University) successfully created a super-resolution “street view” of intracellular organelles’ interactions and has this published in Nature Communications.
This is the first time that researchers get the dynamics of lipid heterogeneity inside subcellular organelles with optical imaging technology. They have developed a Spectrum and Polarization Optical Tomography (SPOT) technique, which can resolve the membrane morphology, polarity, and phase from the intensity, spectrum, and polarization, respectively. Combined with lipophilic probes, the team successfully revealed more than ten types of organelles simultaneously and analyzed their sophisticated lipid dynamics.
A cell is a self-efficient “micro-world” with various organelles responsible for nutrition import, metabolism, endocrine modulation, protein manufacturing, waste transportations, and all the efficient communications. The study of organelles’ dynamics and their interactions will help us understand the cell functions and diagnose the cause of diseases, as well as develop potential drug treatments. As widely-used fluorescence microscopy has limits in the color and type of fluorescent dyes and in temporal and spatial resolutions, it is extremely difficult to get simultaneous imaging of multiple organelle species.
Lipid membranes in subcellular compartments synergistically regulate biophysical membrane properties, membrane protein function, and lipid-protein interactions. Despite its significant role in biochemical function and interaction of subcellular organelles, due to their similar chemical composition, it is challenging to study the classification and interaction of different types of lipid membranes and observe their dynamics for a long time.
Compared with the other existing fluorescence microscopy techniques, SPOT is superior in optical throughput that correlatively obtains the high-dimensional information from six raw images within tens of milliseconds. SPOT’s optical section ability improves the measurement accuracy of polarity and phase. This is the first time that researchers quantitatively study the lipid heterogeneity inside subcellular organelles.
Figure 1 Heterogeneity of subcellular lipid membranes revealed by SPOT.
With SPOT, the lipid heterogeneity on the outer membrane and the cristae of mitochondria, as well as lipid dynamics through endosome maturation, can be clearly imaged in real-time. With the new imaging platform established at SUSTech, researchers observed the multi-organelle interactive activities of cell division, lipid dynamics during the plasma membrane separation, tunneling nanotubules formation, and mitochondrial cristae dissociation.
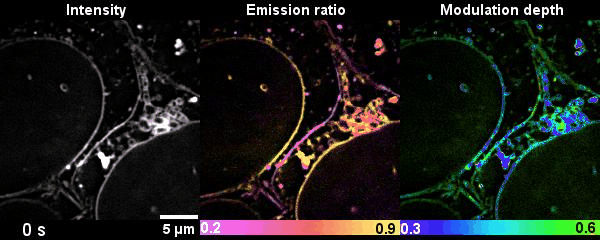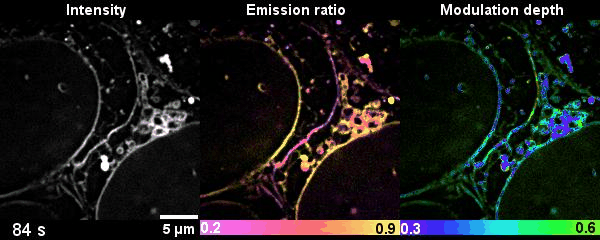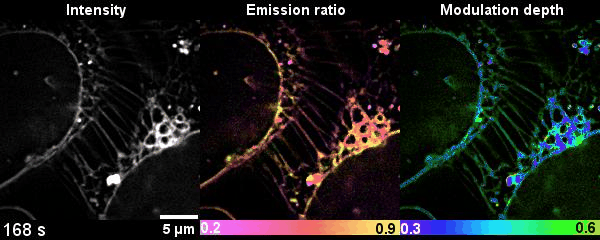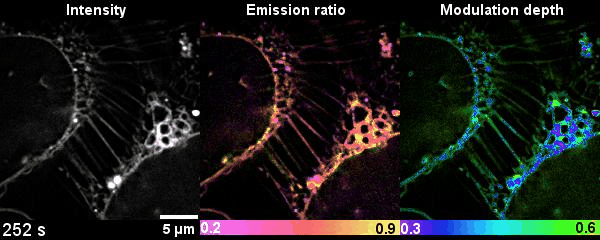
Figure 2 Time-lapse high-dimensional super-resolution imaging of the late-stage division of two U2-OS cells
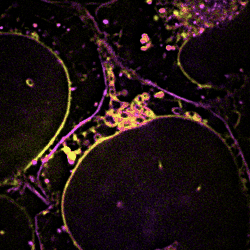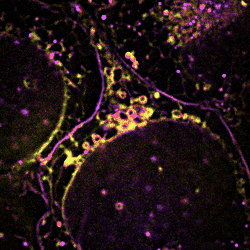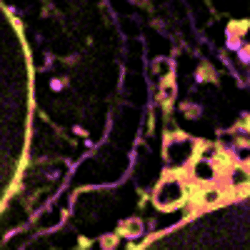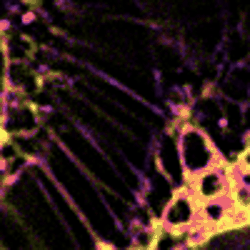
Figure 3 Dynamic changes of lipid membrane during mitochondrial cristae dissociation
Most microscopes can only image four or fewer color channels, far less than the types of intracellular organelles. The synergistic use of membrane morphology, lipid polarity, and the lipid phase can classify more than ten types of organelles.
SUSTech Research Assistant Professor Karl Zhanghao is the co-first author and the co-corresponding author of the work. His contribution includes: (1) Super-resolution Dipole Orientation Mapping (SDOM) technique, (Light: Sci. Appl. 2016, https://doi.org/10.1038/s41467-019-12681-w, highlighted by Nat. Methods, https://doi.org/10.1038/nmeth.4061); (2) polarized Structured Illumination Microscopy (pSIM) technique, (https://10.1038/s41467-019-12681-w, highlighted by Nat. Methods, https://doi.org/10.1038/s41592-019-0682-6); (3) pSIM with improved imaging resolution (Opt. Express, https://doi.org/10.1364/OE.395092), and low-cost SIM with laser interference and digital micro-mirror device (Appl. Phys. Lett. 2020, https://doi.org/10.1063/5.0008264).
Doctoral students Wenhui Liu from Tsinghua University, and Meiqi Li from Peking University are the co-first authors of this work.
This work has been supported by the National Natural Science Foundation of China, National Key Research and Development Program of China, Beijing Natural Science Foundation, and Shenzhen Science and Technology Program.
Article Link:https://doi.org/10.1038/s41467-020-19747-0
Proofread ByEddy Salguero, Yingying XIA, Zhong YANG
Photo By