Researches show that defects in intracellular cargo transport may induce severe neurodegeneration diseases, such as Alzheimer’s disease and Huntington’s disease, which makes the research on the mechanism of intracellular logistics a hotspot in life science.
Recently, a research group led by Dr. Zhiyi Wei and Dr. Cong Yu (Biology, SUStech) published an article in Science Advances, entitled “F-actin disassembly factor MICAL1 binding to Myosin Va mediates cargo unloading during cytokinesis.” Their research reveals a novel mechanism for intracellular cargo transport mediated by cytoskeletal motors.
Similar to the logistics systems in our daily life, intracellular cargo transport is a fundamental cellular process, required for cell growth, division, proliferation, and differentiation. The transportation process is tightly controlled to ensure diverse cargos are delivered at right time to the designated place. In cells, cytoskeletal motors (myosins, kinesins, and dyneins) carry the cargos and deliver them to different destinations for different functions along the cytoskeletons (actin filaments and microtubules) which work as transportation tracks. Defects in intracellular cargo transport may induce severe neurodegeneration diseases, such as Alzheimer’s disease and Huntington’s disease.
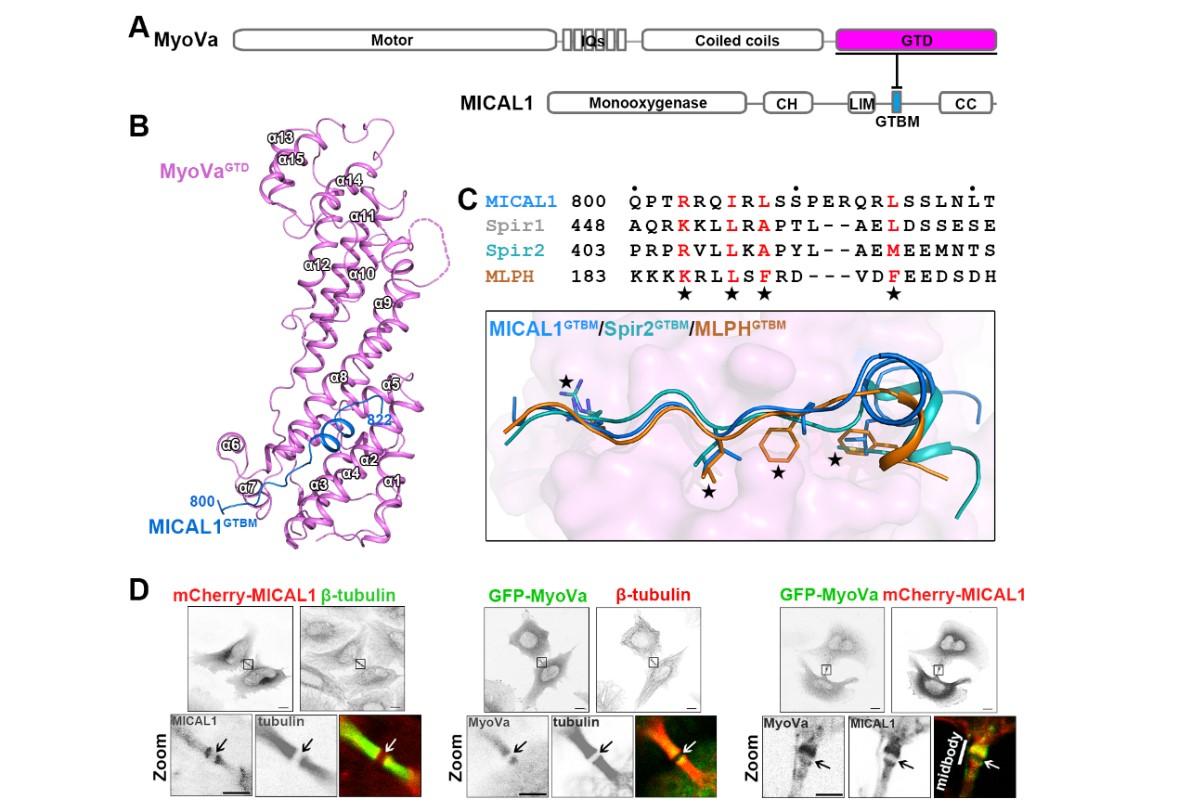
Fig.1 MICAL1 was identified as a novel binding partner of MyoVa at the midbody
To unveil the largely unknown mechanism of intracellular cargo transport, the research team studied MyoVa ( myosin Va ), a classic model for studying cargo transport. MyoVa is an actin-based motor and plays multiple roles in transporting cargo. In this study, MICAL1, a disassembly factor of actin filament, was identified as a new MyoVa-binding partner at the midbody during cytokinesis. The cellular analysis showed that MyoVa is recruited by MICAL1 to promote the unloading of vesicles at the midbody, which provides signal molecules and membrane components for cytokinesis.
Dr. Fengfeng Niu, the first author of the paper, from Dr. Wei’s lab, solved the complex structure of MyoVa and MICAL1 and found that MICAL1 competes with Spire, an assembly factor of actin filament, to interact with MyoVa. Biochemical and cellular experiments collaboratively performed by Dr. Niu and the co-first author Kang Sun in Dr. Yu’s lab further confirmed that MICAL1 and Spire antagonize with each other to control actin polymerization and thereby regulate MyoVa-mediated cargo transport. Based on their findings, the researchers proposed that MyoVa recruits Spire at the starting point to assemble the tracks and initiate transport, and at destinations, MICAL1 outcompetes Spire and then binds with MyoVa to trigger actin tracks disassembly to unload cargoes.
This is the first time that researchers propose that cytoskeletal motors control the loading, transportation, and unloading of intracellular cargo, shedding lights on the spatiotemporal regulation of intracellular cargo transport.
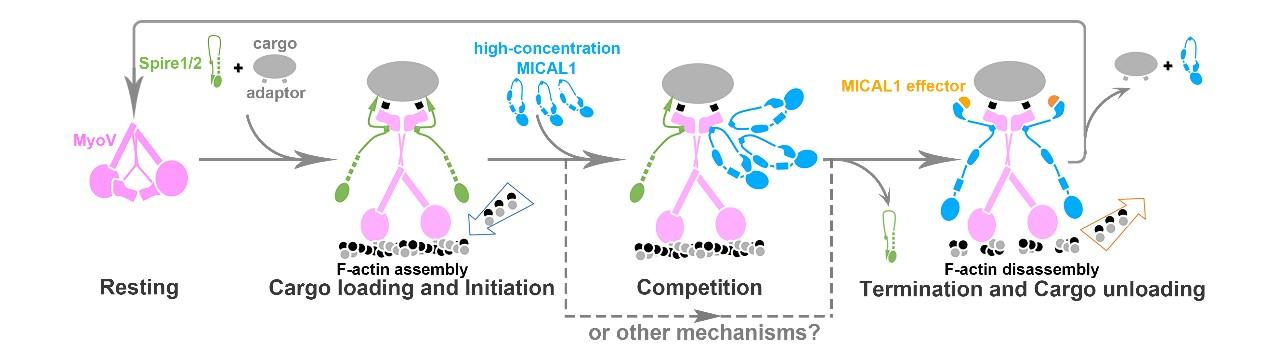
Fig 2. A proposed model for the class V myosin-mediated cargo transport and unloading
Dr. Fengfeng Niu from Dr. Zhiyi Wei’s lab and Kang Sun in Dr. Cong Yu’s lab are the first authors of the paper.
This work is supported by the National Natural Science Foundation of China, Natural Science Foundation of Guangdong Province, Science and Technology Planning Project of Guangdong Province, Shenzhen-Hong Kong Institute of Brain Science, Shenzhen Science and Technology Innovation Commission, Shanghai Synchrotron Radiation Facility (SSRF) and Core Research Facilities at SUSTech.
Link of the research article: https://doi.org/10.1126/sciadv.abb1307
Dr. Zhiyi Wei’s Lab page:http://faculty.sustech.edu.cn/weizy/
Dr. Cong Yu’s Lab page:http://faculty.sustech.edu.cn/yuc/
Proofread ByEddy Salguero, Yingying XIA
Photo By